Membrane-associated zinc peptidase families: comparing ACE and ACE2
- PMID: 16054014
- PMCID: PMC7105243
- DOI: 10.1016/j.bbapap.2004.10.010
Membrane-associated zinc peptidase families: comparing ACE and ACE2
Abstract
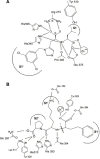
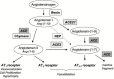
In contrast to the relatively ubiquitous angiotensin-converting enzyme (ACE), expression of the mammalian ACE homologue, ACE2, was initially described in the heart, kidney and testis. ACE2 is a type I integral membrane protein with its active site domain exposed to the extracellular surface of endothelial cells and the renal tubular epithelium. Here ACE2 is poised to metabolise circulating peptides which may include angiotensin II, a potent vasoconstrictor and the product of angiotensin I cleavage by ACE. To this end, ACE2 may counterbalance the effects of ACE within the renin-angiotensin system (RAS). Indeed, ACE2 has been implicated in the regulation of heart and renal function where it is proposed to control the levels of angiotensin II relative to its hypotensive metabolite, angiotensin-(1-7). The recent solution of the structure of ACE2, and ACE, has provided new insight into the substrate and inhibitor profiles of these two key regulators of the RAS. As the complexity of this crucial pathway is unravelled, there is a growing interest in the therapeutic potential of agents that modulate the activity of ACE2.
Figures
Similar articles
-
A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9.Circ Res. 2000 Sep 1;87(5):E1-9. doi: 10.1161/01.res.87.5.e1. Circ Res. 2000. PMID: 10969042
-
Angiotensin-converting enzyme-2: a molecular and cellular perspective.Cell Mol Life Sci. 2004 Nov;61(21):2704-13. doi: 10.1007/s00018-004-4240-7. Cell Mol Life Sci. 2004. PMID: 15549171 Free PMC article. Review.
-
Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells.J Biol Chem. 2005 Nov 25;280(47):39353-62. doi: 10.1074/jbc.M508914200. Epub 2005 Sep 15. J Biol Chem. 2005. PMID: 16166094
-
Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism.Biochem J. 2004 Oct 1;383(Pt 1):45-51. doi: 10.1042/BJ20040634. Biochem J. 2004. PMID: 15283675 Free PMC article.
-
Not just angiotensinases: new roles for the angiotensin-converting enzymes.Cell Mol Life Sci. 2010 Jan;67(1):89-98. doi: 10.1007/s00018-009-0152-x. Epub 2009 Sep 10. Cell Mol Life Sci. 2010. PMID: 19763395 Free PMC article. Review.
Cited by
-
Enzymatic pathways of the brain renin-angiotensin system: unsolved problems and continuing challenges.Regul Pept. 2007 Oct 4;143(1-3):15-27. doi: 10.1016/j.regpep.2007.03.006. Epub 2007 Mar 30. Regul Pept. 2007. PMID: 17493693 Free PMC article. Review.
-
Angiotensin- converting enzyme insertion/deletion polymorphism and its association with coronary artery disease in an Iranian population.J Tehran Heart Cent. 2013 Apr;8(2):89-94. Epub 2013 Apr 28. J Tehran Heart Cent. 2013. PMID: 23967030 Free PMC article.
-
Protein kinase C-regulated aβ production and clearance.Int J Alzheimers Dis. 2011 Jan 17;2011:857368. doi: 10.4061/2011/857368. Int J Alzheimers Dis. 2011. PMID: 21274428 Free PMC article.
-
Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis.Diseases. 2021 Jul 1;9(3):50. doi: 10.3390/diseases9030050. Diseases. 2021. PMID: 34287285 Free PMC article. Review.
-
Dental Healthcare Amid the COVID-19 Pandemic.Int J Environ Res Public Health. 2021 Oct 20;18(21):11008. doi: 10.3390/ijerph182111008. Int J Environ Res Public Health. 2021. PMID: 34769526 Free PMC article. Review.
References
-
- Turner A.J., Hooper N.M. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol. Sci. 2002;23:177. - PubMed
-
- Campbell D.J. Tissue renin–angiotensin system: sites of angiotensin formation. J. Cardiovasc. Pharmacol. 1987;10:S1–S8. - PubMed
-
- Yang H.Y.T., Erdos E.G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim. Biophys. Acta. 1970;214:374–376. - PubMed
-
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homologue of angiotensin-converting enzyme. J. Biol. Chem. 2000;275:33238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

