Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small-molecule CCR5 antagonist
- PMID: 16048963
- PMCID: PMC1196284
- DOI: 10.1128/AAC.49.8.3474-3482.2005
Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small-molecule CCR5 antagonist
Abstract
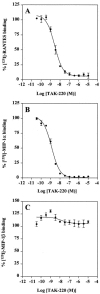
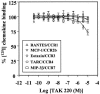
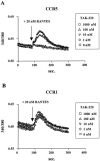
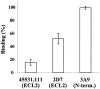
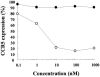
TAK-220 is a member of a novel class of chemokine receptor antagonists and is highly specific to CCR5, as determined by receptor binding and calcium mobilization assays. The compound selectively inhibited coreceptor-mediated entry of human immunodeficiency virus type 1 (HIV-1) into host cells and HIV-1 infection mediated by CCR5. TAK-220 inhibited the replication of six CCR5-using (R5) HIV-1 clinical isolates in peripheral blood mononuclear cells (PBMCs) with a mean 90% effective concentration of 13 nM. The anti-HIV-1 activity of TAK-220 was not affected by addition of high concentrations of human serum. It equally inhibited R5 HIV-1 replication in PBMCs obtained from eight different donors, irrespective of the levels of viral production. Furthermore, the anti-HIV-1 activity of TAK-220 was found to be subtype independent. TAK-220 did not induce CCR5 internalization but blocked the binding of two monoclonal antibodies that recognize the second extracellular loop of CCR5 in CCR5-expressing cells. These results suggest that TAK-220 selectively inhibits R5 HIV-1 replication by interfering with coreceptor-mediated entry of the virus into host cells. At a dose of 5 mg/kg of body weight, TAK-220 showed oral bioavailabilities of 9.5 and 28.9% in rats and monkeys, respectively. Thus, TAK-220 is a promising candidate for the treatment of HIV-1 infection.
Figures
Similar articles
-
TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans.Antimicrob Agents Chemother. 2005 Nov;49(11):4584-91. doi: 10.1128/AAC.49.11.4584-4591.2005. Antimicrob Agents Chemother. 2005. PMID: 16251299 Free PMC article. Clinical Trial.
-
Inhibitory effects of small-molecule CCR5 antagonists on human immunodeficiency virus type 1 envelope-mediated membrane fusion and viral replication.Antimicrob Agents Chemother. 2001 Dec;45(12):3538-43. doi: 10.1128/AAC.45.12.3538-3543.2001. Antimicrob Agents Chemother. 2001. PMID: 11709336 Free PMC article.
-
Highly potent and orally active CCR5 antagonists as anti-HIV-1 agents: synthesis and biological activities of 1-benzazocine derivatives containing a sulfoxide moiety.J Med Chem. 2006 Mar 23;49(6):2037-48. doi: 10.1021/jm0509703. J Med Chem. 2006. PMID: 16539392
-
[Chemokine receptors and its importance in the replication cycle of human immunodeficiency virus: clinical and therapeutic implications].Acta Med Port. 2008 Sep-Oct;21(5):497-504. Epub 2009 Jan 16. Acta Med Port. 2008. PMID: 19187693 Review. Portuguese.
-
HIV chemokine receptor inhibitors as novel anti-HIV drugs.Cytokine Growth Factor Rev. 2005 Dec;16(6):659-77. doi: 10.1016/j.cytogfr.2005.05.009. Epub 2005 Jul 6. Cytokine Growth Factor Rev. 2005. PMID: 16005254 Review.
Cited by
-
TAK-220, a novel small-molecule CCR5 antagonist, has favorable anti-human immunodeficiency virus interactions with other antiretrovirals in vitro.Antimicrob Agents Chemother. 2005 Aug;49(8):3483-5. doi: 10.1128/AAC.49.8.3483-3485.2005. Antimicrob Agents Chemother. 2005. PMID: 16048964 Free PMC article.
-
Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity.J Hepatol. 2017 Apr;66(4):685-692. doi: 10.1016/j.jhep.2016.11.009. Epub 2016 Nov 25. J Hepatol. 2017. PMID: 27890789 Free PMC article.
-
Inhibition of Inflammatory and Neuropathic Pain by Targeting a Mu Opioid Receptor/Chemokine Receptor5 Heteromer (MOR-CCR5).J Med Chem. 2015 Nov 12;58(21):8647-57. doi: 10.1021/acs.jmedchem.5b01245. Epub 2015 Oct 20. J Med Chem. 2015. PMID: 26451468 Free PMC article.
-
TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans.Antimicrob Agents Chemother. 2005 Nov;49(11):4584-91. doi: 10.1128/AAC.49.11.4584-4591.2005. Antimicrob Agents Chemother. 2005. PMID: 16251299 Free PMC article. Clinical Trial.
-
A bivalent compound targeting CCR5 and the mu opioid receptor treats inflammatory arthritis pain in mice without inducing pharmacologic tolerance.Arthritis Res Ther. 2018 Jul 27;20(1):154. doi: 10.1186/s13075-018-1661-5. Arthritis Res Ther. 2018. PMID: 30053832 Free PMC article.
References
-
- Aarons, E. J., S. Beddows, T. Willinghaml Wu, and R. A. Koup. 2001. Adaptation to blockade of human immunodeficiency virus type 1 entry imposed by the anti-CCR5 monoclonal antibody 2D7. Virology 287:382-390. - PubMed
-
- Alkhatib, G., S. S. Ahuja, D. Light, S. Mummidi, E. A. Berger, and S. K. Ahuja. 1997. CC chemokine receptor 5-mediated signaling and HIV-1 co-receptor activity share common structural determinants. Critical residues in the third extracellular loop support HIV-1 fusion. J. Biol. Chem. 272:19771-19776. - PubMed
-
- Baba, M., H. Miyake, M. Okamoto, Y. Iizawa, and K. Okonogi. 2000. Establishment of a CCR5-expressing T-lymphoblastoid cell line highly susceptible to R5 HIV type 1. AIDS Res. Hum. Retrovir. 16:935-941. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

