Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils
- PMID: 16043520
- PMCID: PMC2213086
- DOI: 10.1084/jem.20050778
Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils
Abstract
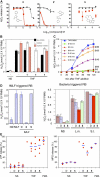
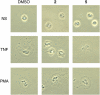
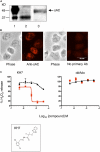
Through chemical screening, we identified a pyrazolone that reversibly blocked the activation of phagocyte oxidase (phox) in human neutrophils in response to tumor necrosis factor (TNF) or formylated peptide. The pyrazolone spared activation of phox by phorbol ester or bacteria, bacterial killing, TNF-induced granule exocytosis and phox assembly, and endothelial transmigration. We traced the pyrazolone's mechanism of action to inhibition of TNF-induced intracellular Ca2+ elevations, and identified a nontransmembrane ("soluble") adenylyl cyclase (sAC) in neutrophils as a Ca2+-sensing source of cAMP. A sAC inhibitor mimicked the pyrazolone's effect on phox. Both compounds blocked TNF-induced activation of Rap1A, a phox-associated guanosine triphosphatase that is regulated by cAMP. Thus, TNF turns on phox through a Ca2+-triggered, sAC-dependent process that may involve activation of Rap1A. This pathway may offer opportunities to suppress oxidative damage during inflammation without blocking antimicrobial function.
Figures



Similar articles
-
Chemical inhibitors of TNF signal transduction in human neutrophils point to distinct steps in cell activation.J Leukoc Biol. 2006 Jan;79(1):147-54. doi: 10.1189/jlb.0605308. Epub 2005 Nov 7. J Leukoc Biol. 2006. PMID: 16275893
-
Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1.J Biol Chem. 2006 Jun 23;281(25):17253-17258. doi: 10.1074/jbc.M603500200. Epub 2006 Apr 20. J Biol Chem. 2006. PMID: 16627466 Free PMC article.
-
Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation.J Exp Med. 2003 Jan 6;197(1):63-75. doi: 10.1084/jem.20021638. J Exp Med. 2003. PMID: 12515814 Free PMC article.
-
Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines.Vet Immunol Immunopathol. 2011 Sep 15;143(1-2):1-10. doi: 10.1016/j.vetimm.2011.05.037. Epub 2011 Jun 23. Vet Immunol Immunopathol. 2011. PMID: 21764141 Review.
-
Store-operated Ca²⁺-entry and adenylyl cyclase.Cell Calcium. 2015 Oct;58(4):368-75. doi: 10.1016/j.ceca.2015.04.004. Epub 2015 Apr 22. Cell Calcium. 2015. PMID: 25978874 Review.
Cited by
-
Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling.J Cell Biol. 2016 Jul 18;214(2):181-95. doi: 10.1083/jcb.201512075. Epub 2016 Jul 11. J Cell Biol. 2016. PMID: 27402953 Free PMC article.
-
Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation.J Biol Chem. 2010 Sep 24;285(39):29998-30007. doi: 10.1074/jbc.M110.113621. Epub 2010 Jul 16. J Biol Chem. 2010. PMID: 20639512 Free PMC article.
-
Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells.J Gen Physiol. 2008 Sep;132(3):329-38. doi: 10.1085/jgp.200810044. Epub 2008 Aug 11. J Gen Physiol. 2008. PMID: 18695009 Free PMC article.
-
Bicarbonate, carbon dioxide and pH sensing via mammalian bicarbonate-regulated soluble adenylyl cyclase.Interface Focus. 2021 Apr 6;11(2):20200034. doi: 10.1098/rsfs.2020.0034. Epub 2021 Feb 12. Interface Focus. 2021. PMID: 33633833 Free PMC article. Review.
-
Somatic 'soluble' adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex 'knockout' mice.PLoS One. 2008 Sep 22;3(9):e3251. doi: 10.1371/journal.pone.0003251. PLoS One. 2008. PMID: 18806876 Free PMC article.
References
-
- Weiss, S.J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365–376. - PubMed
-
- Fang, F.C. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820–832. - PubMed
-
- Reeves, E.P., H. Lu, H.L. Jacobs, C.G. Messina, S. Bolsover, G. Gabella, E.O. Potma, A. Warley, J. Roes, and A.W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297. - PubMed
-
- Weiss, S.J., G. Peppin, X. Ortiz, C. Ragsdale, and S.T. Test. 1985. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 227:747–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

