A local mechanism mediates NAD-dependent protection of axon degeneration
- PMID: 16043516
- PMCID: PMC2171458
- DOI: 10.1083/jcb.200504028
A local mechanism mediates NAD-dependent protection of axon degeneration
Abstract
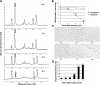
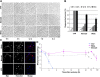
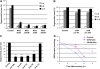
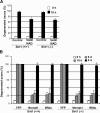
Axon degeneration occurs frequently in neurodegenerative diseases and peripheral neuropathies. Important insight into the mechanisms of axon degeneration arose from findings that the degeneration of transected axons is delayed in Wallerian degeneration slow (Wlds) mice with the overexpression of a fusion protein with the nicotinamide adenine dinucleotide (NAD) synthetic enzyme, nicotinamide mononucleotide adenylyltransferase (Nmnat1). Although both Wld(s) and Nmnat1 themselves are functional in preventing axon degeneration in neuronal cultures, the underlying mechanism for Nmnat1- and NAD-mediated axon protection remains largely unclear. We demonstrate that NAD levels decrease in degenerating axons and that preventing this axonal NAD decline efficiently protects axons from degeneration. In support of a local protective mechanism, we show that the degeneration of axonal segments that have been separated from their soma could be prevented by the exogenous application of NAD or its precursor nicotinamide. Furthermore, we provide evidence that such Nmnat1/NAD-mediated protection is primarily mediated by their effects on local bioenergetics. Together, our results suggest a novel molecular pathway for axon degeneration.
Figures

Similar articles
-
NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration.Cell Death Differ. 2007 Jan;14(1):116-27. doi: 10.1038/sj.cdd.4401944. Epub 2006 Apr 28. Cell Death Differ. 2007. PMID: 16645633
-
The Wlds transgene reduces axon loss in a Charcot-Marie-Tooth disease 1A rat model and nicotinamide delays post-traumatic axonal degeneration.Neurobiol Dis. 2011 Apr;42(1):1-8. doi: 10.1016/j.nbd.2010.12.006. Epub 2010 Dec 16. Neurobiol Dis. 2011. PMID: 21168501
-
Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo.J Neurosci. 2010 Oct 6;30(40):13291-304. doi: 10.1523/JNEUROSCI.1189-10.2010. J Neurosci. 2010. PMID: 20926655 Free PMC article.
-
Axon degeneration: Mechanisms and implications of a distinct program from cell death.Neurochem Int. 2010 Mar;56(4):529-34. doi: 10.1016/j.neuint.2010.01.013. Epub 2010 Feb 1. Neurochem Int. 2010. PMID: 20117162 Review.
-
NAD and axon degeneration: from the Wlds gene to neurochemistry.Cell Adh Migr. 2009 Jan-Mar;3(1):77-87. doi: 10.4161/cam.3.1.7483. Epub 2009 Jan 25. Cell Adh Migr. 2009. PMID: 19372760 Free PMC article. Review.
Cited by
-
Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection.Front Cell Neurosci. 2013 Jan 9;6:60. doi: 10.3389/fncel.2012.00060. eCollection 2012. Front Cell Neurosci. 2013. PMID: 23316132 Free PMC article.
-
Insulin/IGF-I and related signaling pathways regulate aging in nondividing cells: from yeast to the mammalian brain.ScientificWorldJournal. 2010 Jan 21;10:161-77. doi: 10.1100/tsw.2010.8. ScientificWorldJournal. 2010. PMID: 20098959 Free PMC article. Review.
-
Differential effects of SARM1 inhibition in traumatic glaucoma and EAE optic neuropathies.Mol Ther Nucleic Acids. 2023 Feb 27;32:13-27. doi: 10.1016/j.omtn.2023.02.029. eCollection 2023 Jun 13. Mol Ther Nucleic Acids. 2023. PMID: 36950280 Free PMC article.
-
The purine nucleosides adenosine and guanosine delay axonal degeneration in vitro.J Neurochem. 2009 Apr;109(2):595-602. doi: 10.1111/j.1471-4159.2009.06002.x. Epub 2009 Feb 20. J Neurochem. 2009. PMID: 19245660 Free PMC article.
-
Preserve and protect: maintaining axons within functional circuits.Trends Neurosci. 2014 Oct;37(10):572-82. doi: 10.1016/j.tins.2014.07.007. Epub 2014 Aug 26. Trends Neurosci. 2014. PMID: 25167775 Free PMC article. Review.
References
-
- Araki, T., Y. Sasaki, and J. Milbrandt. 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 305:1010–1013. - PubMed
-
- Blander, G., and L. Guarente. 2004. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73:417–435. - PubMed
-
- Brunet, A., L.B. Sweeney, J.F. Sturgill, K.F. Chua, P.L. Greer, Y. Lin, H. Tran, S.E. Ross, R. Mostoslavsky, H.Y. Cohen, et al. 2004. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 303:2011–2015. - PubMed
-
- Coleman, M.P., and V.H. Perry. 2002. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 25:532–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

