Meiotic telomere clustering requires actin for its formation and cohesin for its resolution
- PMID: 16027219
- PMCID: PMC2171397
- DOI: 10.1083/jcb.200501042
Meiotic telomere clustering requires actin for its formation and cohesin for its resolution
Abstract
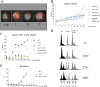
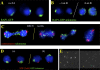
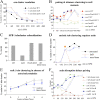
In diploid organisms, meiosis reduces the chromosome number by half during the formation of haploid gametes. During meiotic prophase, telomeres transiently cluster at a limited sector of the nuclear envelope (bouquet stage) near the spindle pole body (SPB). Cohesin is a multisubunit complex that contributes to chromosome segregation in meiosis I and II divisions. In yeast meiosis, deficiency for Rec8 cohesin subunit induces telomere clustering to persist, whereas telomere cluster-SPB colocalization is defective. These defects are rescued by expressing the mitotic cohesin Scc1 in rec8delta meiosis, whereas bouquet-stage exit is independent of Cdc5 pololike kinase. An analysis of living Saccharomyces cerevisiae meiocytes revealed highly mobile telomeres from leptotene up to pachytene, with telomeres experiencing an actin- but not microtubule-dependent constraint of mobility during the bouquet stage. Our results suggest that cohesin is required for exit from actin polymerization-dependent telomere clustering and for linking the SPB to the telomere cluster in synaptic meiosis.
Figures
Similar articles
-
Chromosome morphogenesis: condensin-dependent cohesin removal during meiosis.Cell. 2005 Nov 4;123(3):397-407. doi: 10.1016/j.cell.2005.09.014. Cell. 2005. PMID: 16269332
-
Increased ploidy and KAR3 and SIR3 disruption alter the dynamics of meiotic chromosomes and telomeres.J Cell Sci. 2003 Jun 15;116(Pt 12):2431-42. doi: 10.1242/jcs.00453. Epub 2003 May 6. J Cell Sci. 2003. PMID: 12734403
-
Set1- and Clb5-deficiencies disclose the differential regulation of centromere and telomere dynamics in Saccharomyces cerevisiae meiosis.J Cell Sci. 2005 Nov 1;118(Pt 21):4985-94. doi: 10.1242/jcs.02612. J Cell Sci. 2005. PMID: 16254243
-
Telomere attachment and clustering during meiosis.Cell Mol Life Sci. 2007 Jan;64(2):117-24. doi: 10.1007/s00018-006-6463-2. Cell Mol Life Sci. 2007. PMID: 17219025 Free PMC article. Review.
-
Tying SUMO modifications to dynamic behaviors of chromosomes during meiotic prophase of Saccharomyces cerevisiae.J Biomed Sci. 2007 Jul;14(4):481-90. doi: 10.1007/s11373-007-9176-0. Epub 2007 May 25. J Biomed Sci. 2007. PMID: 17530453 Review.
Cited by
-
Chromosome-nuclear envelope tethering - a process that orchestrates homologue pairing during plant meiosis?J Cell Sci. 2020 Aug 12;133(15):jcs243667. doi: 10.1242/jcs.243667. J Cell Sci. 2020. PMID: 32788229 Free PMC article. Review.
-
CDK Regulation of Meiosis: Lessons from S. cerevisiae and S. pombe.Genes (Basel). 2020 Jun 29;11(7):723. doi: 10.3390/genes11070723. Genes (Basel). 2020. PMID: 32610611 Free PMC article. Review.
-
The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis.Nat Cell Biol. 2014 Feb;16(2):145-56. doi: 10.1038/ncb2896. Epub 2014 Jan 12. Nat Cell Biol. 2014. PMID: 24413433
-
The Diverse Cellular Functions of Inner Nuclear Membrane Proteins.Cold Spring Harb Perspect Biol. 2021 Sep 1;13(9):a040477. doi: 10.1101/cshperspect.a040477. Cold Spring Harb Perspect Biol. 2021. PMID: 33753404 Free PMC article. Review.
-
Nuclear actin extends, with no contraction in sight.Mol Biol Cell. 2005 Nov;16(11):5055-60. doi: 10.1091/mbc.e05-07-0656. Epub 2005 Sep 7. Mol Biol Cell. 2005. PMID: 16148048 Free PMC article. Review.
References
-
- Abe, T., K. Takano, A. Suzuki, Y. Shimada, M. Inagaki, N. Sato, T. Obinata, and T. Endo. 2004. Myocyte differentiation generates nuclear invaginations traversed by myofibrils associating with sarcomeric protein mRNAs. J. Cell Sci. 117:6523–6534. - PubMed
-
- Armstrong, S.J., A.P. Caryl, G.H. Jones, and F.C. Franklin. 2002. Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J. Cell Sci. 115:3645–3655. - PubMed
-
- Bannister, L.A., L.G. Reinholdt, R.J. Munroe, and J.C. Schimenti. 2004. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 40:184–194. - PubMed
-
- Bergerat, A., B. de Massy, D. Gadelle, P.C. Varoutas, A. Nicolas, and P. Forterre. 1997. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 386:414–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

