Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers
- PMID: 16014928
- PMCID: PMC1181598
- DOI: 10.1128/JVI.79.15.9665-9676.2005
Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers
Abstract
The recently emerged severe acute respiratory syndrome coronavirus (SARS-CoV) is a potent pathogen of humans and is capable of rapid global spread. Peptide-conjugated antisense morpholino oligomers (P-PMO) were designed to bind by base pairing to specific sequences in the SARS-CoV (Tor2 strain) genome. The P-PMO were tested for their capacity to inhibit production of infectious virus as well as to probe the function of conserved viral RNA motifs and secondary structures. Several virus-targeted P-PMO and a random-sequence control P-PMO showed low inhibitory activity against SARS coronavirus. Certain other virus-targeted P-PMO reduced virus-induced cytopathology and cell-to-cell spread as a consequence of decreasing viral amplification. Active P-PMO were effective when administered at any time prior to peak viral synthesis and exerted sustained antiviral effects while present in culture medium. P-PMO showed low nonspecific inhibitory activity against translation of nontargeted RNA or growth of the arenavirus lymphocytic choriomeningitis virus. Two P-PMO targeting the viral transcription-regulatory sequence (TRS) region in the 5' untranslated region were the most effective inhibitors tested. After several viral passages in the presence of a TRS-targeted P-PMO, partially drug-resistant SARS-CoV mutants arose which contained three contiguous base point mutations at the binding site of a TRS-targeted P-PMO. Those partially resistant viruses grew more slowly and formed smaller plaques than wild-type SARS-CoV. These results suggest PMO compounds have powerful therapeutic and investigative potential toward coronavirus infection.
Figures
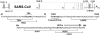


Similar articles
-
Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers.Antimicrob Agents Chemother. 2006 Nov;50(11):3724-33. doi: 10.1128/AAC.00644-06. Epub 2006 Sep 11. Antimicrob Agents Chemother. 2006. PMID: 16966399 Free PMC article.
-
In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus.Antimicrob Agents Chemother. 2007 Jul;51(7):2470-82. doi: 10.1128/AAC.00069-07. Epub 2007 May 7. Antimicrob Agents Chemother. 2007. PMID: 17485503 Free PMC article.
-
Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication.J Virol. 2005 Apr;79(8):4599-609. doi: 10.1128/JVI.79.8.4599-4609.2005. J Virol. 2005. PMID: 15795246 Free PMC article.
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
-
Inhibition and escape of SARS-CoV treated with antisense morpholino oligomers.Adv Exp Med Biol. 2006;581:567-71. doi: 10.1007/978-0-387-33012-9_103. Adv Exp Med Biol. 2006. PMID: 17037599 Free PMC article. Review. No abstract available.
Cited by
-
Advanced morpholino oligomers: a novel approach to antiviral therapy.Antiviral Res. 2012 Apr;94(1):80-8. doi: 10.1016/j.antiviral.2012.02.004. Epub 2012 Feb 14. Antiviral Res. 2012. PMID: 22353544 Free PMC article. Review.
-
A Defective Viral Particle Approach to COVID-19.Cells. 2022 Jan 17;11(2):302. doi: 10.3390/cells11020302. Cells. 2022. PMID: 35053418 Free PMC article. Review.
-
Bacterial resistance to antisense peptide phosphorodiamidate morpholino oligomers.Antimicrob Agents Chemother. 2012 Dec;56(12):6147-53. doi: 10.1128/AAC.00850-12. Epub 2012 Sep 17. Antimicrob Agents Chemother. 2012. PMID: 22985881 Free PMC article.
-
Exploring IRES region accessibility by interference of foot-and-mouth disease virus infectivity.PLoS One. 2012;7(7):e41382. doi: 10.1371/journal.pone.0041382. Epub 2012 Jul 18. PLoS One. 2012. PMID: 22815996 Free PMC article.
-
Gene-specific countermeasures against Ebola virus based on antisense phosphorodiamidate morpholino oligomers.PLoS Pathog. 2006 Jan;2(1):e1. doi: 10.1371/journal.ppat.0020001. Epub 2006 Jan 13. PLoS Pathog. 2006. PMID: 16415982 Free PMC article.
References
-
- Bacha, U., J. Barrila, A. Velazquez-Campoy, S. A. Leavitt, and E. Freire. 2004. Identification of novel inhibitors of the SARS coronavirus main protease 3CLpro. Biochemistry 43:4906-4912. - PubMed
-
- Benedetto, A., G. B. Rossi, C. Amici, F. Belardelli, L. Cioe, G. Carruba, and L. Carrasco. 1980. Inhibition of animal virus production by means of translation inhibitors unable to penetrate normal cells. Virology 106:123-132. - PubMed
-
- Bosch, B. J., B. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. Osterhaus, and P. J. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 101:8455-8460. - PMC - PubMed
-
- Buchmeier, M. J., J. H. Elder, and M. B. Oldstone. 1978. Protein structure of lymphocytic choriomeningitis virus: identification of the virus structural and cell associated polypeptides. Virology 89:133-145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

