The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria
- PMID: 16009724
- PMCID: PMC2171414
- DOI: 10.1083/jcb.200503148
The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria
Abstract
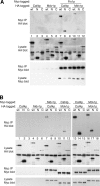
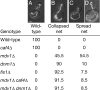
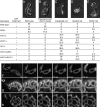
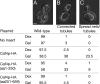
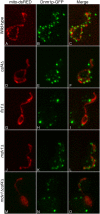
The mitochondrial division machinery regulates mitochondrial dynamics and consists of Fis1p, Mdv1p, and Dnm1p. Mitochondrial division relies on the recruitment of the dynamin-related protein Dnm1p to mitochondria. Dnm1p recruitment depends on the mitochondrial outer membrane protein Fis1p. Mdv1p interacts with Fis1p and Dnm1p, but is thought to act at a late step during fission because Mdv1p is dispensable for Dnm1p localization. We identify the WD40 repeat protein Caf4p as a Fis1p-associated protein that localizes to mitochondria in a Fis1p-dependent manner. Caf4p interacts with each component of the fission apparatus: with Fis1p and Mdv1p through its NH2-terminal half and with Dnm1p through its COOH-terminal WD40 domain. We demonstrate that mdv1delta yeast contain residual mitochondrial fission due to the redundant activity of Caf4p. Moreover, recruitment of Dnm1p to mitochondria is disrupted in mdv1delta caf4delta yeast, demonstrating that Mdv1p and Caf4p are molecular adaptors that recruit Dnm1p to mitochondrial fission sites. Our studies support a revised model for assembly of the mitochondrial fission apparatus.
Figures

Similar articles
-
The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission.J Cell Biol. 2002 Aug 5;158(3):445-52. doi: 10.1083/jcb.200205031. Epub 2002 Aug 5. J Cell Biol. 2002. PMID: 12163467 Free PMC article.
-
Fis1p and Caf4p, but not Mdv1p, determine the polar localization of Dnm1p clusters on the mitochondrial surface.J Cell Sci. 2006 Aug 1;119(Pt 15):3098-106. doi: 10.1242/jcs.03026. Epub 2006 Jul 11. J Cell Sci. 2006. PMID: 16835275
-
Dnm1p-dependent peroxisome fission requires Caf4p, Mdv1p and Fis1p.J Cell Sci. 2008 May 15;121(Pt 10):1633-40. doi: 10.1242/jcs.026344. Epub 2008 Apr 29. J Cell Sci. 2008. PMID: 18445678 Free PMC article.
-
Molecular machinery of mitochondrial dynamics in yeast.Biol Chem. 2007 Sep;388(9):917-26. doi: 10.1515/BC.2007.110. Biol Chem. 2007. PMID: 17696775 Review.
-
The machines that divide and fuse mitochondria.Annu Rev Biochem. 2007;76:751-80. doi: 10.1146/annurev.biochem.76.071905.090048. Annu Rev Biochem. 2007. PMID: 17362197 Review.
Cited by
-
Molecular mechanisms of mitochondrial dynamics.Nat Rev Mol Cell Biol. 2024 Oct 17. doi: 10.1038/s41580-024-00785-1. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39420231 Review.
-
The Mitochondrial Distribution and Morphology Family 33 Gene FgMDM33 Is Involved in Autophagy and Pathogenesis in Fusarium graminearum.J Fungi (Basel). 2024 Aug 16;10(8):579. doi: 10.3390/jof10080579. J Fungi (Basel). 2024. PMID: 39194905 Free PMC article.
-
Biased placement of Mitochondria fission facilitates asymmetric inheritance of protein aggregates during yeast cell division.PLoS Comput Biol. 2023 Nov 27;19(11):e1011588. doi: 10.1371/journal.pcbi.1011588. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 38011208 Free PMC article.
-
Biogenesis, inheritance, and 3D ultrastructure of the microsporidian mitosome.Life Sci Alliance. 2023 Oct 30;7(1):e202201635. doi: 10.26508/lsa.202201635. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37903625 Free PMC article.
-
Convergent and divergent mechanisms of peroxisomal and mitochondrial division.J Cell Biol. 2023 Sep 4;222(9):e202304076. doi: 10.1083/jcb.202304076. Epub 2023 Aug 2. J Cell Biol. 2023. PMID: 37530713 Free PMC article.
References
-
- Denis, C.L., and J. Chen. 2003. The CCR4-NOT complex plays diverse roles in mRNA metabolism. Prog. Nucleic Acid Res. Mol. Biol. 73:221–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

