Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis
- PMID: 15994795
- PMCID: PMC1168792
- DOI: 10.1128/JVI.79.14.9019-9025.2005
Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis
Abstract
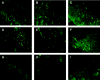
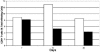
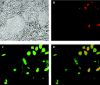
Upon infection of murine trigeminal ganglia with herpes simplex virus type 1 (HSV-1), an immune response is initiated resulting in significant infiltration of CD8+ T cells. Previous investigators have observed a lack of apoptosis in HSV-1 trigeminal ganglia even in the presence of cytotoxic immune cells. To determine the role of the latency-associated transcript (LAT) in inhibiting apoptosis, we examined mice during acute and latent infection with HSV-1 (strain 17 or a LAT-negative deletion mutant strain 17 N/H) by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) and fluorescence-activated cell sorting (FACS). FACS analysis revealed CD8+ T cells in the trigeminal ganglia by day 7, with more being present in 17- than 17 N/H-infected trigeminal ganglia (6.22% versus 3.5%) and a decrease in number through day 30 (2.7% to 1.2%). To detect apoptotic CD8+ T cells, sections were assayed by TUNEL and stained for CD8+ T cells. By day 7, approximately 10% of CD8+ T cells in both 17- and 17 N/H-infected trigeminal ganglia had undergone apoptosis. By day 30, 58% and 74% of all T cells had undergone apoptosis in 17- and 17 N/H-infected trigeminal ganglia, respectively. Furthermore, no HSV strain 17-infected trigeminal ganglion neurons were apoptotic, but 0.087% of 17deltaSty and 0.98% of 17 N/H-infected neurons were apoptotic. We conclude that the antiapoptotic effect of LAT appears to require the LAT promoter, with most of the antiapoptotic effect mapping within the StyI (+447) to the HpaI (+1667) region and a minor contribution from the upstream StyI (+76) to StyI (+447) region.
Figures






Similar articles
-
Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia.Virology. 1996 Nov 1;225(1):72-81. doi: 10.1006/viro.1996.0576. Virology. 1996. PMID: 8918535
-
Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons.Virology. 2000 Dec 20;278(2):309-21. doi: 10.1006/viro.2000.0678. Virology. 2000. PMID: 11118355
-
Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia.J Neuropathol Exp Neurol. 2006 Oct;65(10):1022-30. doi: 10.1097/01.jnen.0000235852.92963.bf. J Neuropathol Exp Neurol. 2006. PMID: 17021407
-
Immunity in latent Herpes simplex virus infection.Acta Virol. 2005;49(3):159-67. Acta Virol. 2005. PMID: 16178513 Review.
-
Immune control of herpes simplex virus during latency.Curr Opin Immunol. 2004 Aug;16(4):463-9. doi: 10.1016/j.coi.2004.05.003. Curr Opin Immunol. 2004. PMID: 15245740 Review.
Cited by
-
Small non-coding RNAs encoded within the herpes simplex virus type 1 latency associated transcript (LAT) cooperate with the retinoic acid inducible gene I (RIG-I) to induce beta-interferon promoter activity and promote cell survival.Virus Res. 2013 Aug;175(2):101-9. doi: 10.1016/j.virusres.2013.04.005. Epub 2013 May 3. Virus Res. 2013. PMID: 23648811 Free PMC article.
-
A Journey through the Minefield of the Discovery and Characterization of Latency-Related RNA/Latency-Associated Transcript.Viruses. 2024 Sep 30;16(10):1562. doi: 10.3390/v16101562. Viruses. 2024. PMID: 39459896 Free PMC article. Review.
-
Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle.Interdiscip Perspect Infect Dis. 2010;2010:262415. doi: 10.1155/2010/262415. Epub 2010 Feb 15. Interdiscip Perspect Infect Dis. 2010. PMID: 20169002 Free PMC article.
-
Identification of herpes simplex virus type 1 proteins encoded within the first 1.5 kb of the latency-associated transcript.J Neurovirol. 2009 Sep;15(5-6):439-48. doi: 10.3109/13550280903296353. J Neurovirol. 2009. PMID: 20175695
-
Herpes simplex virus 1 regulates β-catenin expression in TG neurons during the latency-reactivation cycle.PLoS One. 2020 Mar 30;15(3):e0230870. doi: 10.1371/journal.pone.0230870. eCollection 2020. PLoS One. 2020. PMID: 32226020 Free PMC article.
References
-
- Asano, S., T. Honda, F. Goshima, D. Watanabe, Y. Miyake, Y. Sugiura, and Y. Nishiyama. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51-56. - PubMed
-
- Bak, I. J., C. H. Markham, M. L. Cook, and J. G. Stevens. 1977. Intraaxonal transport of herpes simplex virus in the rat central nervous system. Brain Res. 136:415-429. - PubMed
-
- Block, T. M., S. Deshmane, J. Masonis, J. Maggioncalda, T. Valyi-Nagi, and N. M. Fraser. 1993. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology 192:618-630. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

