Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication
- PMID: 15994774
- PMCID: PMC1168730
- DOI: 10.1128/JVI.79.14.8802-8811.2005
Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication
Abstract
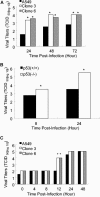
The induction of apoptotic cell death is a hallmark of influenza virus infection. Although a variety of cellular and viral proteins have been implicated in this process, to date no conserved cellular pathway has been identified. In this study, we report that the tumor suppressor protein p53 is essential for the induction of cell death in influenza virus-infected cells. In primary human lung cells, influenza virus increased p53 protein levels. This was also noted in the human lung cell line A549, along with the up-regulation of p53-dependent gene transcription. Reduction of p53 activity in A549 cells inhibited influenza virus-induced cell death as measured by trypan blue exclusion and caspase activity. These findings were not cell type specific. Influenza virus-induced cell death was absent in mouse embryo fibroblasts isolated from p53 knockout mice, which was not the case in wild-type mouse embryo fibroblasts, suggesting that p53 is a common cellular pathway leading to influenza virus-induced cell death. Surprisingly, inhibiting p53 activity led to elevated virus replication. Mechanistically, this may be due to the decrease in interferon signaling in p53-deficient cells, suggesting that functional p53 is involved in the interferon response to influenza infection. To our knowledge, these are the first studies demonstrating that p53 is involved in influenza virus-induced cell death and that inhibiting p53 leads to increased viral titers, potentially through modulation of the interferon response.
Figures

Similar articles
-
Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling.Apoptosis. 2007 Aug;12(8):1419-32. doi: 10.1007/s10495-007-0071-y. Apoptosis. 2007. PMID: 17468837
-
Cellular co-infection can modulate the efficiency of influenza A virus production and shape the interferon response.PLoS Pathog. 2020 Oct 16;16(10):e1008974. doi: 10.1371/journal.ppat.1008974. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33064776 Free PMC article.
-
The Nonstructural NS1 Protein of Influenza Viruses Modulates TP53 Splicing through Host Factor CPSF4.J Virol. 2019 Mar 21;93(7):e02168-18. doi: 10.1128/JVI.02168-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651364 Free PMC article.
-
Cellular networks involved in the influenza virus life cycle.Cell Host Microbe. 2010 Jun 25;7(6):427-39. doi: 10.1016/j.chom.2010.05.008. Cell Host Microbe. 2010. PMID: 20542247 Free PMC article. Review.
-
Transport of the influenza virus genome from nucleus to nucleus.Viruses. 2013 Oct 2;5(10):2424-46. doi: 10.3390/v5102424. Viruses. 2013. PMID: 24104053 Free PMC article. Review.
Cited by
-
Host cell p53 associates with the feline calicivirus major viral capsid protein VP1, the protease-polymerase NS6/7, and the double-stranded RNA playing a role in virus replication.Virology. 2020 Nov;550:78-88. doi: 10.1016/j.virol.2020.08.008. Epub 2020 Aug 27. Virology. 2020. PMID: 32890980 Free PMC article.
-
Cellular microRNA let-7c inhibits M1 protein expression of the H1N1 influenza A virus in infected human lung epithelial cells.J Cell Mol Med. 2012 Oct;16(10):2539-46. doi: 10.1111/j.1582-4934.2012.01572.x. J Cell Mol Med. 2012. PMID: 22452878 Free PMC article.
-
Identification of Nifurtimox and Chrysin as Anti-Influenza Virus Agents by Clinical Transcriptome Signature Reversion.Int J Mol Sci. 2022 Feb 21;23(4):2372. doi: 10.3390/ijms23042372. Int J Mol Sci. 2022. PMID: 35216485 Free PMC article.
-
DNA mismatch repair is required for the host innate response and controls cellular fate after influenza virus infection.Nat Microbiol. 2019 Nov;4(11):1964-1977. doi: 10.1038/s41564-019-0509-3. Epub 2019 Jul 29. Nat Microbiol. 2019. PMID: 31358986 Free PMC article.
-
Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation.J Biol Chem. 2015 Nov 13;290(46):28001-17. doi: 10.1074/jbc.M115.669465. Epub 2015 Oct 7. J Biol Chem. 2015. PMID: 26446794 Free PMC article.
References
-
- Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. - PubMed
-
- Barnes, B. J., M. J. Kellum, K. E. Pinder, J. A. Frisancho, and P. M. Pitha. 2003. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 63:6424-6431. - PubMed
-
- Blaho, J. A. 2003. Virus infection and apoptosis (issue 1). Dedicated to the memory of Lois K. Miller. Int. Rev. Immunol. 22:321-326. - PubMed
-
- Blaho, J. A. 2004. Virus infection and apoptosis (issue II) an introduction: cheating death or death as a fact of life? Int. Rev. Immunol. 23:1-6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

