Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine
- PMID: 15980868
- PMCID: PMC1576256
- DOI: 10.1038/sj.bjp.0706315
Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine
Abstract
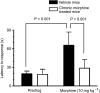
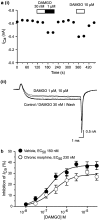
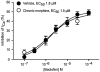
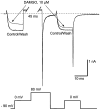
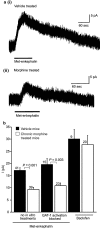
The midbrain periaqueductal gray (PAG) is a major site of opioid analgesic action, and a significant site of cellular adaptations to chronic morphine treatment (CMT). We examined mu-opioid receptor (MOP) regulation of voltage-gated calcium channel currents (I(Ca)) and G-protein-activated K channel currents (GIRK) in PAG neurons from CMT mice. Mice were injected s.c. with 300 mg kg(-1) of morphine base in a slow release emulsion three times over 5 days, or with emulsion alone (vehicles). This protocol produced significant tolerance to the antinociceptive effects of morphine in a test of thermal nociception. Voltage clamp recordings were made of I(Ca) in acutely isolated PAG neurons and GIRK in PAG slices. The MOP agonist DAMGO (Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin) inhibited I(Ca) in neurons from CMT mice (230 nM) with a similar potency to vehicle (150 nM), but with a reduced maximal effectiveness (37% inhibition in vehicle neurons, 27% in CMT neurons). Inhibition of I(Ca) by the GABA(B) agonist baclofen was not altered by CMT. Met-enkephalin-activated GIRK currents recorded in PAG slices were significantly smaller in neurons from CMT mice than vehicles, while GIRK currents activated by baclofen were unaltered. These data demonstrate that CMT-induced antinociceptive tolerance is accompanied by homologous reduction in the effectiveness of MOP agonists to inhibit I(Ca) and activate GIRK. Thus, a reduction in MOP number and/or functional coupling to G proteins accompanies the characteristic cellular adaptations to CMT previously described in PAG neurons.
Figures
Similar articles
-
Decreased mu-opioid receptor signalling and a reduction in calcium current density in sensory neurons from chronically morphine-treated mice.Br J Pharmacol. 2006 Aug;148(7):947-55. doi: 10.1038/sj.bjp.0706820. Epub 2006 Jun 19. Br J Pharmacol. 2006. PMID: 16783402 Free PMC article.
-
RGS14 prevents morphine from internalizing Mu-opioid receptors in periaqueductal gray neurons.Cell Signal. 2007 Dec;19(12):2558-71. doi: 10.1016/j.cellsig.2007.08.003. Epub 2007 Aug 15. Cell Signal. 2007. PMID: 17825524
-
Mu-opioid receptor modulation of calcium channel current in periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1.Br J Pharmacol. 1999 Apr;126(7):1553-8. doi: 10.1038/sj.bjp.0702457. Br J Pharmacol. 1999. PMID: 10323586 Free PMC article.
-
The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain.Eur J Pharmacol. 2013 Sep 15;716(1-3):94-105. doi: 10.1016/j.ejphar.2013.01.066. Epub 2013 Mar 13. Eur J Pharmacol. 2013. PMID: 23499699 Review.
-
Efficacy and ligand bias at the μ-opioid receptor.Br J Pharmacol. 2013 Aug;169(7):1430-46. doi: 10.1111/bph.12222. Br J Pharmacol. 2013. PMID: 23646826 Free PMC article. Review.
Cited by
-
Chronic morphine induces adaptations in opioid receptor signaling in a thalamo-cortico-striatal circuit that are projection-dependent, sex-specific and regulated by mu opioid receptor phosphorylation.bioRxiv [Preprint]. 2023 Feb 14:2023.02.13.528057. doi: 10.1101/2023.02.13.528057. bioRxiv. 2023. Update in: J Neurosci. 2024 Jan 17;44(3):e0293232023. doi: 10.1523/JNEUROSCI.0293-23.2023 PMID: 36824766 Free PMC article. Updated. Preprint.
-
Key differences in regulation of opioid receptors localized to presynaptic terminals compared to somas: Relevance for novel therapeutics.Neuropharmacology. 2023 Mar 15;226:109408. doi: 10.1016/j.neuropharm.2022.109408. Epub 2022 Dec 28. Neuropharmacology. 2023. PMID: 36584882 Free PMC article. Review.
-
Chronic Morphine Induces Adaptations in Opioid Receptor Signaling in a Thalamostriatal Circuit That Are Location Dependent, Sex Specific, and Regulated by μ-Opioid Receptor Phosphorylation.J Neurosci. 2024 Jan 17;44(3):e0293232023. doi: 10.1523/JNEUROSCI.0293-23.2023. J Neurosci. 2024. PMID: 37985179 Free PMC article.
-
Phosphorylation of unique C-terminal sites of the mu-opioid receptor variants 1B2 and 1C1 influences their Gs association following chronic morphine.J Neurochem. 2020 Feb;152(4):449-467. doi: 10.1111/jnc.14863. Epub 2019 Oct 20. J Neurochem. 2020. PMID: 31479519 Free PMC article.
-
Effect of heat shock protein 70 modulators on the development of morphine analgesic tolerance in rats.Behav Pharmacol. 2020 Apr;31(2&3):179-185. doi: 10.1097/FBP.0000000000000528. Behav Pharmacol. 2020. PMID: 31770112 Free PMC article.
References
-
- AKIL H., MAYER D.J., LIEBESKIND J.C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191:961–962. - PubMed
-
- BAGLEY E.E., GERKE M.E., VAUGHAN C.W., HACK S.P., CHRISTIE M.J. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005;45:433–445. - PubMed
-
- BELLCHAMBERS C.E., CHIENG B., KEAY K.A., CHRISTIE M.J. Swim-stress but not opioid withdrawal increases expression of c-fos immunoreactivity in rat periaqueductal gray neurons which project to the rostral ventromedial medulla. Neuroscience. 1998;83:517–524. - PubMed
-
- BERNSTEIN M.S., WELCH S.P. μ-opioid receptor downregulation and cAMP-dependent protein kinase phosphorylation in a mouse model of chronic morphine tolerance. Mol. Brain Res. 1998;55:237–242. - PubMed
-
- BOHN L.M., GAINETDINOV R.R., LIN F.-T., LEFKOWITZ R.J., CARON M.G. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

