Pressure perturbation and differential scanning calorimetric studies of bipolar tetraether liposomes derived from the thermoacidophilic archaeon Sulfolobus acidocaldarius
- PMID: 15980181
- PMCID: PMC1366687
- DOI: 10.1529/biophysj.105.063933
Pressure perturbation and differential scanning calorimetric studies of bipolar tetraether liposomes derived from the thermoacidophilic archaeon Sulfolobus acidocaldarius
Abstract
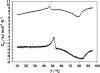
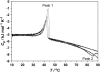
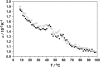
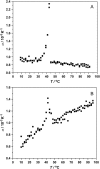
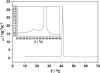
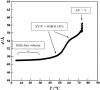
Differential scanning calorimetry (DSC) and pressure perturbation calorimetry (PPC) were used to characterize thermal phase transitions, membrane packing, and volumetric properties in multilamellar vesicles (MLVs) composed of the polar lipid fraction E (PLFE) isolated from the thermoacidophilic archaeon Sulfolobus acidocaldarius grown at different temperatures. For PLFE MLVs derived from cells grown at 78 degrees C, the first DSC heating scan exhibits an endothermic transition at 46.7 degrees C, a small hump near 60 degrees C, and a broad exothermic transition at 78.5 degrees C, whereas the PPC scan reveals two transitions at approximately 45 degrees C and 60 degrees C. The endothermic peak at 46.7 degrees C is attributed to a lamellar-to-lamellar phase transition and has an unusually low DeltaH (3.5 kJ/mol) and DeltaV/V (0.1%) value, as compared to those for the main phase transitions of saturated diacyl monopolar diester lipids. This result may arise from the restricted trans-gauche conformational changes in the dibiphytanyl chain due to the presence of cyclopentane rings and branched methyl groups and due to the spanning of the lipid molecules over the whole membrane. The exothermic peak at 78.5 degrees C probably corresponds to a lamellar-to-cubic phase transition and exhibits a large and negative DeltaH value (-23.2 kJ/mol), which is uncommon for normal lamellar-to-cubic phospholipid phase transformations. This exothermic transition disappears in the subsequent heating scans and thus may involve a metastable phase, which is irreversible at the scan rate used. Further, there is no distinct peak in the plot of the thermal expansion coefficient alpha versus temperature near 78.5 degrees C, indicating that this lamellar-to-cubic phase transition is not accompanied by any significant volume change. For PLFE MLVs derived from cells grown at 65 degrees C, similar DSC and PPC profiles and thermal history responses were obtained. However, the lower growth temperature yields a higher DeltaV/V ( approximately 0.25%) and DeltaH (14 kJ/mol) value for the lamellar-to-lamellar phase transition measured at the same pH (2.1). A lower growth temperature also generates a less negative temperature dependence of alpha. The changes in DeltaV/V, DeltaH, and the temperature dependence of alpha can be attributed to the decrease in the number of cyclopentane rings in PLFE at the lower growth temperature. The relatively low DeltaV/V and small DeltaH involved in the phase transitions help to explain why PLFE liposomes are remarkably thermally stable and also echo the proposal that PLFE liposomes are generally rigid and tightly packed. These results help us to understand why, despite the occurrence of thermal-induced phase transitions, PLFE liposomes exhibit a remarkably low temperature sensitivity of proton permeation and dye leakage.
Figures

Similar articles
-
Low permeability of liposomal membranes composed of bipolar tetraether lipids from thermoacidophilic archaebacterium Sulfolobus acidocaldarius.Biochemistry. 1998 Jan 6;37(1):107-15. doi: 10.1021/bi972163e. Biochemistry. 1998. PMID: 9425030
-
Two-photon fluorescence microscopy studies of bipolar tetraether giant liposomes from thermoacidophilic archaebacteria Sulfolobus acidocaldarius.Biophys J. 2000 Jul;79(1):416-25. doi: 10.1016/S0006-3495(00)76303-X. Biophys J. 2000. PMID: 10866967 Free PMC article.
-
Physical properties of archaeal tetraether lipid membranes as revealed by differential scanning and pressure perturbation calorimetry, molecular acoustics, and neutron reflectometry: effects of pressure and cell growth temperature.Langmuir. 2012 Mar 20;28(11):5211-7. doi: 10.1021/la300142r. Epub 2012 Mar 7. Langmuir. 2012. PMID: 22352806
-
Differential Scanning Calorimetry (DSC): a tool to study the thermal behavior of lipid bilayers and liposomal stability.J Liposome Res. 2008;18(3):159-73. doi: 10.1080/08982100802310261. J Liposome Res. 2008. PMID: 18770070 Review.
-
Differential scanning calorimetry in drug-membrane interactions.Biochem Biophys Res Commun. 2024 May 21;709:149806. doi: 10.1016/j.bbrc.2024.149806. Epub 2024 Mar 19. Biochem Biophys Res Commun. 2024. PMID: 38579619 Review.
Cited by
-
Biological Membranes in Extreme Conditions: Simulations of Anionic Archaeal Tetraether Lipid Membranes.PLoS One. 2016 May 11;11(5):e0155287. doi: 10.1371/journal.pone.0155287. eCollection 2016. PLoS One. 2016. PMID: 27167213 Free PMC article.
-
On physical properties of tetraether lipid membranes: effects of cyclopentane rings.Archaea. 2012;2012:138439. doi: 10.1155/2012/138439. Epub 2012 Sep 18. Archaea. 2012. PMID: 23028246 Free PMC article. Review.
-
Polar Lipid Fraction E from Sulfolobus acidocaldarius and Dipalmitoylphosphatidylcholine Can Form Stable yet Thermo-Sensitive Tetraether/Diester Hybrid Archaeosomes with Controlled Release Capability.Int J Mol Sci. 2020 Nov 9;21(21):8388. doi: 10.3390/ijms21218388. Int J Mol Sci. 2020. PMID: 33182284 Free PMC article.
-
Compressibilities and volume fluctuations of archaeal tetraether liposomes.Biophys J. 2010 Nov 17;99(10):3319-26. doi: 10.1016/j.bpj.2010.09.061. Biophys J. 2010. PMID: 21081080 Free PMC article.
-
Liquid but durable: molecular dynamics simulations explain the unique properties of archaeal-like membranes.Sci Rep. 2014 Dec 12;4:7462. doi: 10.1038/srep07462. Sci Rep. 2014. PMID: 25501042 Free PMC article.
References
-
- Langworthy, T. A., and J. L. Pond. 1986. Membranes and lipids of thermophiles. In Thermophiles: General, Molecular, and Applied Microbiology. T. D. Brock, editor. John Wiley & Sons, New York. 107–134.
-
- Kates, M. 1992. Archaebacterial lipids: structure, biosynthesis and function. In The Archaebacteria: Biochemistry and Biotechnology. M. J. Danson, D. W. Hough, and G. G. Lunt, editors. Portland Press, London. 51–72. - PubMed
-
- Lo, S.-L., and E. L. Chang. 1990. Purification and characterization of a liposomal-forming tetraether lipid fraction. Biochem. Biophys. Res. Commun. 167:238–243. - PubMed
-
- Sugai, A., R. Sakuma, I. Fukuda, N. Kurosawa, Y. H. Itoh, K. Kon, S. Ando, and T. Itoh. 1995. The structure of the core polyol of the ether lipids from Sulfolobus acidocaldarius. Lipids. 30:339–344. - PubMed
-
- Gliozzi, A., A. Relini, and P. L.-G. Chong. 2002. Structure and permeability properties of biomimetic membranes of bolaform archaeal tetraether lipids. J. Membr. Sci. 206:131–147.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

