Development of a new vaccine for the prevention of Lassa fever
- PMID: 15971954
- PMCID: PMC1160587
- DOI: 10.1371/journal.pmed.0020183
Development of a new vaccine for the prevention of Lassa fever
Abstract
Background: Recent importation of Lassa fever into Germany, the Netherlands, the United Kingdom, and the United States by travelers on commercial airlines from Africa underscores the public health challenge of emerging viruses. Currently, there are no licensed vaccines for Lassa fever, and no experimental vaccine has completely protected nonhuman primates against a lethal challenge.
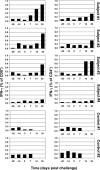
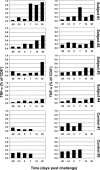
Methods and findings: We developed a replication-competent vaccine against Lassa virus based on attenuated recombinant vesicular stomatitis virus vectors expressing the Lassa viral glycoprotein. A single intramuscular vaccination of the Lassa vaccine elicited a protective immune response in nonhuman primates against a lethal Lassa virus challenge. Vaccine shedding was not detected in the monkeys, and none of the animals developed fever or other symptoms of illness associated with vaccination. The Lassa vaccine induced strong humoral and cellular immune responses in the four vaccinated and challenged monkeys. Despite a transient Lassa viremia in vaccinated animals 7 d after challenge, the vaccinated animals showed no evidence of clinical disease. In contrast, the two control animals developed severe symptoms including rashes, facial edema, and elevated liver enzymes, and ultimately succumbed to the Lassa infection.
Conclusion: Our data suggest that the Lassa vaccine candidate based on recombinant vesicular stomatitis virus is safe and highly efficacious in a relevant animal model that faithfully reproduces human disease.
Conflict of interest statement
Figures

Comment in
-
Some tolerance for fur--animal studies in PLoS Medicine.PLoS Med. 2005 Jun;2(6):e203. doi: 10.1371/journal.pmed.0020203. Epub 2005 Jun 28. PLoS Med. 2005. PMID: 15971961 Free PMC article. No abstract available.
Similar articles
-
Effective vaccine for lassa fever.J Virol. 2000 Aug;74(15):6777-83. doi: 10.1128/jvi.74.15.6777-6783.2000. J Virol. 2000. PMID: 10888616 Free PMC article.
-
A Vaccine Platform against Arenaviruses Based on a Recombinant Hyperattenuated Mopeia Virus Expressing Heterologous Glycoproteins.J Virol. 2018 May 29;92(12):e02230-17. doi: 10.1128/JVI.02230-17. Print 2018 Jun 15. J Virol. 2018. PMID: 29593043 Free PMC article.
-
Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses.Emerg Infect Dis. 2015 Feb;21(2):305-7. doi: 10.3201/eid2102.141649. Emerg Infect Dis. 2015. PMID: 25625358 Free PMC article.
-
Vaccine platforms to control Lassa fever.Expert Rev Vaccines. 2016 Sep;15(9):1135-50. doi: 10.1080/14760584.2016.1184575. Epub 2016 May 24. Expert Rev Vaccines. 2016. PMID: 27136941 Review.
-
Towards a human Lassa fever vaccine.Rev Med Virol. 2001 Sep-Oct;11(5):331-41. doi: 10.1002/rmv.329. Rev Med Virol. 2001. PMID: 11590670 Review.
Cited by
-
Immune responses and Lassa virus infection.Viruses. 2012 Nov 5;4(11):2766-85. doi: 10.3390/v4112766. Viruses. 2012. PMID: 23202504 Free PMC article. Review.
-
Arenavirus Genome Rearrangement for the Development of Live Attenuated Vaccines.J Virol. 2015 Jul;89(14):7373-84. doi: 10.1128/JVI.00307-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972555 Free PMC article.
-
Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine.J Virol. 2006 Oct;80(19):9659-66. doi: 10.1128/JVI.00959-06. J Virol. 2006. PMID: 16973570 Free PMC article.
-
T cell-dependence of Lassa fever pathogenesis.PLoS Pathog. 2010 Mar 26;6(3):e1000836. doi: 10.1371/journal.ppat.1000836. PLoS Pathog. 2010. PMID: 20360949 Free PMC article.
-
A recombinant vesicular stomatitis-based Lassa fever vaccine elicits rapid and long-term protection from lethal Lassa virus infection in guinea pigs.NPJ Vaccines. 2019 Feb 8;4:8. doi: 10.1038/s41541-019-0104-x. eCollection 2019. NPJ Vaccines. 2019. PMID: 30774999 Free PMC article.
References
-
- Fisher-Hoch SP, McCormick JB. Lassa fever vaccine: A review. Expert Rev Vaccines. 2004;3:103–111. - PubMed
-
- McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–444. - PubMed
-
- Communicable Disease Surveillance Centre. Lassa fever imported to England. Commun Dis Rep CDR Weekly. 2000;10:99. - PubMed
-
- Schmitz H, Kohler B, Laue T, Drosten C, Veldkamp PJ, et al. Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect. 2002;4:43–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

