Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice
- PMID: 15964995
- PMCID: PMC1151660
- DOI: 10.1101/gad.1299305
Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice
Abstract
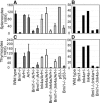
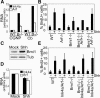
The Polycomb group (PcG) gene Bmi1 promotes cell proliferation and stem cell self-renewal by repressing the Ink4a/Arf locus. We used a genetic approach to investigate whether Ink4a or Arf is more critical for relaying Bmi1 function in lymphoid cells, neural progenitors, and neural stem cells. We show that Arf is a general target of Bmi1, however particularly in neural stem cells, derepression of Ink4a contributes to Bmi1(-/-) phenotypes. Additionally, we demonstrate haploinsufficient effects for the Ink4a/Arf locus downstream of Bmi1 in vivo. This suggests differential, cell type-specific roles for Ink4a versus Arf in PcG-mediated (stem) cell cycle control.
Figures
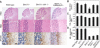
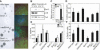
Similar articles
-
Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways.Genes Dev. 2005 Jun 15;19(12):1432-7. doi: 10.1101/gad.1299505. Genes Dev. 2005. PMID: 15964994 Free PMC article.
-
Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma.Cancer Cell. 2007 Oct;12(4):328-41. doi: 10.1016/j.ccr.2007.08.032. Cancer Cell. 2007. PMID: 17936558
-
Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice.J Exp Med. 2006 Oct 2;203(10):2247-53. doi: 10.1084/jem.20052477. Epub 2006 Sep 5. J Exp Med. 2006. PMID: 16954369 Free PMC article.
-
Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors.Cold Spring Harb Symp Quant Biol. 2005;70:177-85. doi: 10.1101/sqb.2005.70.057. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869752 Review.
-
Bmi1, stem cells, and senescence regulation.J Clin Invest. 2004 Jan;113(2):175-9. doi: 10.1172/JCI20800. J Clin Invest. 2004. PMID: 14722607 Free PMC article. Review.
Cited by
-
Bmi1 limits dilated cardiomyopathy and heart failure by inhibiting cardiac senescence.Nat Commun. 2015 Mar 9;6:6473. doi: 10.1038/ncomms7473. Nat Commun. 2015. Retraction in: Nat Commun. 2017 Mar 07;8:14006. doi: 10.1038/ncomms14006. PMID: 25751743 Free PMC article. Retracted.
-
Control of stress signaling in stem cells: crossroads of stem cells and cancer.Tumour Biol. 2016 Oct;37(10):12983-12990. doi: 10.1007/s13277-016-5249-x. Epub 2016 Jul 27. Tumour Biol. 2016. PMID: 27460084 Review.
-
Targeting Enhancer of Zeste Homolog 2 as a promising strategy for cancer treatment.World J Clin Oncol. 2016 Apr 10;7(2):135-48. doi: 10.5306/wjco.v7.i2.135. World J Clin Oncol. 2016. PMID: 27081636 Free PMC article.
-
Epigenetic regulation of pancreatic islets.Curr Diab Rep. 2013 Oct;13(5):624-32. doi: 10.1007/s11892-013-0403-y. Curr Diab Rep. 2013. PMID: 23907485 Review.
-
Temporal and epigenetic regulation of neurodevelopmental plasticity.Philos Trans R Soc Lond B Biol Sci. 2008 Jan 12;363(1489):23-38. doi: 10.1098/rstb.2006.2010. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 17311782 Free PMC article. Review.
References
-
- Akasaka T., Kanno, M., Balling, R., Mieza, M.A., Taniguchi, M., and Koseki, H. 1996. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122: 1513-1522. - PubMed
-
- Baptista C.A., Hatten, M.E., Blazeski, R., and Mason, C.A. 1994. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron 12: 243-260. - PubMed
-
- Core N., Bel, S., Gaunt, S.J., Aurrand-Lions, M., Pearce, J., Fisher, A., and Djabali, M. 1997. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development 124: 721-729. - PubMed
-
- Core N., Joly, F., Boned, A., and Djabali, M. 2004. Disruption of E2F signaling suppresses the INK4a-induced proliferative defect in M33-deficient mice. Oncogene 23: 7660-7668. - PubMed
-
- Dahmane N. and Ruiz-i-Altaba, A. 1999. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126: 3089-3100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
