Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation
- PMID: 15958750
- PMCID: PMC6724870
- DOI: 10.1523/JNEUROSCI.0599-05.2005
Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation
Abstract
The maintenance of long-term potentiation (LTP) requires a brief period of accelerated protein synthesis soon after synaptic stimulation, suggesting that an early phase of enhanced translation contributes to stable LTP. The mechanism regulating protein synthesis and the location and identities of mRNAs translated are not well understood. Here, we show in acute brain slices that the induction of protein synthesis-dependent hippocampal LTP increases the expression of elongation factor 1A (eEF1A), the mRNA of which contains a 5' terminal oligopyrimidine tract. This effect is blocked by rapamycin, indicating that the increase in EF1A expression is mediated by the mammalian target of rapamycin (mTOR) pathway. We find that mRNA for eEF1A is present in pyramidal cell dendrites and that the LTP-associated increase in eEF1A expression was intact in dendrites that had been severed from their cell bodies before stimulation. eEF1A levels increased within 5 min after stimulation in a translation-dependent manner, and this effect remained stable for 3 h. These results suggest a mechanism whereby synaptic stimulation, by signaling through the mTOR pathway, produces an increase in dendritic translational capacity that contributes to LTP maintenance.
Figures


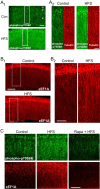
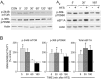
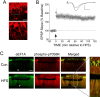
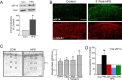
Similar articles
-
Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway.J Neurosci. 2007 May 30;27(22):5885-94. doi: 10.1523/JNEUROSCI.4548-06.2007. J Neurosci. 2007. PMID: 17537959 Free PMC article.
-
Forskolin induction of late-LTP and up-regulation of 5' TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells.J Neurochem. 2008 Aug;106(3):1160-74. doi: 10.1111/j.1471-4159.2008.05470.x. Epub 2008 May 9. J Neurochem. 2008. PMID: 18466337 Free PMC article.
-
A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus.Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):467-72. doi: 10.1073/pnas.012605299. Epub 2001 Dec 26. Proc Natl Acad Sci U S A. 2002. PMID: 11756682 Free PMC article.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
Modulating macroautophagy: a neuronal perspective.Future Med Chem. 2012 Sep;4(13):1715-31. doi: 10.4155/fmc.12.112. Future Med Chem. 2012. PMID: 22924509 Free PMC article. Review.
-
The role of altered translation in intellectual disability and epilepsy.Prog Neurobiol. 2022 Jun;213:102267. doi: 10.1016/j.pneurobio.2022.102267. Epub 2022 Mar 29. Prog Neurobiol. 2022. PMID: 35364140 Free PMC article. Review.
-
Spatially restricting gene expression by local translation at synapses.Trends Neurosci. 2010 Apr;33(4):173-82. doi: 10.1016/j.tins.2010.01.005. Epub 2010 Mar 19. Trends Neurosci. 2010. PMID: 20303187 Free PMC article. Review.
-
Axonal mRNA in uninjured and regenerating cortical mammalian axons.J Neurosci. 2009 Apr 15;29(15):4697-707. doi: 10.1523/JNEUROSCI.6130-08.2009. J Neurosci. 2009. PMID: 19369540 Free PMC article.
-
Striatal proteomic analysis suggests that first L-dopa dose equates to chronic exposure.PLoS One. 2008 Feb 13;3(2):e1589. doi: 10.1371/journal.pone.0001589. PLoS One. 2008. PMID: 18270577 Free PMC article.
References
-
- Biberman Y, Meyuhas O (1997) Substitution of just five nucleotides at and around the transcription start site of rat β-actin promoter is sufficient to render the resulting transcript a subject for translational control. FEBS Lett 405: 333-336. - PubMed
-
- Chan P, Gonzalez-Maeso J, Ruf F, Bishop DF, Hof PR, Sealfon SC (2005) Epsilon sarcoglycan immunoreactiviy and mRNA expression in mouse brain. J Comp Neurol 482: 50-73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
