Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis
- PMID: 15951544
- PMCID: PMC1774598
- DOI: 10.1136/gut.2004.052316
Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis
Abstract
Background and aims: We examined the hypothesis of an anti-inflammatory effect of phosphatidylcholine in ulcerative colitis.
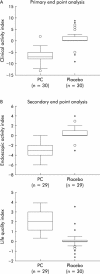
Methods: A phase IIA, double blind, randomised, placebo controlled study was performed in 60 patients with chronic active, non steroid dependent, ulcerative colitis, with a clinical activity index (CAI) of > or = 4. Retarded release phosphatidylcholine rich phospholipids and placebo were administered at a dose of 6 g daily over three months. The primary end point was a change in CAI towards clinical remission (CAI < or = 3) or CAI improvement by > or = 50%. Secondary end points included > or = 50% changes in endoscopic activity index (EAI), histology, and quality of life scores.
Results: Induction of clinical remission (CAI < or = 3) as the primary outcome variable was attained by 16 (53%) patients in the phosphatidylcholine treated group compared with three (10%) in the placebo group (p<0.00001). The rate of clinical remission and CAI improvement was 90% in the phosphatidylcholine group and only 10% in the placebo group. A median drop of seven points in the CAI score (70% improvement) was recorded in the phosphatidylcholine group compared with no change in the placebo group. Secondary end point analysis revealed concomitant drops in EAI and histology scores (p = 0.00016 and p = 0.0067 compared with placebo, respectively). Improvement in quality of life was reported by 16 of 29 evaluated patients in the phosphatidylcholine group compared with two of 30 in the placebo group (p = 0.00005).
Conclusion: Retarded release oral phosphatidylcholine is effective in alleviating inflammatory activity caused by ulcerative colitis.
Figures
Comment in
-
Reinforcing the mucus: a new therapeutic approach for ulcerative colitis?Gut. 2005 Jul;54(7):900-3. doi: 10.1136/gut.2004.058453. Gut. 2005. PMID: 15951531 Free PMC article. Review.
Similar articles
-
Delayed release phosphatidylcholine in chronic-active ulcerative colitis: a randomized, double-blinded, dose finding study.J Clin Gastroenterol. 2010 May-Jun;44(5):e101-7. doi: 10.1097/MCG.0b013e3181c29860. J Clin Gastroenterol. 2010. PMID: 20048683 Clinical Trial.
-
Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis.Aliment Pharmacol Ther. 2004 Apr 1;19(7):739-47. doi: 10.1111/j.1365-2036.2004.01902.x. Aliment Pharmacol Ther. 2004. PMID: 15043514 Clinical Trial.
-
Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group.Am J Gastroenterol. 1993 Aug;88(8):1188-97. Am J Gastroenterol. 1993. PMID: 8338086 Clinical Trial.
-
A review of infliximab use in ulcerative colitis.Clin Ther. 2008 Feb;30(2):223-30. doi: 10.1016/j.clinthera.2008.02.014. Clin Ther. 2008. PMID: 18343261 Review.
-
Delayed release phosphatidylcholine as new therapeutic drug for ulcerative colitis--a review of three clinical trials.Expert Opin Investig Drugs. 2010 Dec;19(12):1623-30. doi: 10.1517/13543784.2010.535514. Expert Opin Investig Drugs. 2010. PMID: 21105858 Review.
Cited by
-
Probiotic pre-administration reduces mortality in a mouse model of cecal ligation and puncture-induced sepsis.Exp Ther Med. 2016 Sep;12(3):1836-1842. doi: 10.3892/etm.2016.3534. Epub 2016 Jul 20. Exp Ther Med. 2016. PMID: 27588102 Free PMC article.
-
Exogenous sphingomyelinase causes impaired intestinal epithelial barrier function.World J Gastroenterol. 2007 Oct 21;13(39):5217-25. doi: 10.3748/wjg.v13.i39.5217. World J Gastroenterol. 2007. PMID: 17876892 Free PMC article.
-
Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics.Front Immunol. 2013 Sep 17;4:280. doi: 10.3389/fimmu.2013.00280. Front Immunol. 2013. PMID: 24062746 Free PMC article. Review.
-
Increased basolateral sorting of carcinoembryonic antigen in a polarized colon carcinoma cell line after cholesterol depletion-Implications for treatment of inflammatory bowel disease.World J Gastroenterol. 2008 Mar 14;14(10):1528-33. doi: 10.3748/wjg.14.1528. World J Gastroenterol. 2008. PMID: 18330942 Free PMC article.
-
First multicenter study of modified release phosphatidylcholine "LT-02" in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses.Am J Gastroenterol. 2014 Jul;109(7):1041-51. doi: 10.1038/ajg.2014.104. Epub 2014 May 6. Am J Gastroenterol. 2014. PMID: 24796768 Free PMC article. Clinical Trial.
References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. - PubMed
-
- Podolsky D. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. - PubMed
-
- Veltkamp C, Tonkonogy SL, De Jong YP, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology 2001;120:900–13. - PubMed
-
- Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 1995;57:565–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
