Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies
- PMID: 15948039
- PMCID: PMC1242175
- DOI: 10.1007/s11095-005-4590-3
Tumor-targeted gene delivery using poly(ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies
Abstract
Purpose: To develop safe and effective systemically administered nonviral gene therapy vectors for solid tumors, DNA-containing poly(ethylene glycol)-modified (PEGylated) gelatin nanoparticles were fabricated and evaluated in vitro and in vivo.
Methods: Reporter plasmid DNA encoding for beta-galactosidase (pCMV-beta) was encapsulated in gelatin and PEGylated gelatin nanoparticles using a water-ethanol solvent displacement method under controlled pH and temperature. Lewis lung carcinoma (LLC) cells in culture were transfected with the pCMV-beta in the control and nanoparticle formulations. Periodically, the expression of beta-galactosidase in the cells was measured quantitatively using an enzymatic assay for the conversion of o-nitrophenyl-beta-D: -galactopyranoside (ONPG) to o-nitrophenol (ONP). Qualitative expression of beta-galactosidase in LLC cells was observed by staining with 5-bromo-4-chloro-3-indolyl-beta-D: -galactopyranoside (X-gal). Additionally, the plasmid DNA-encapsulated gelatin and PEGylated gelatin nanoparticles were administered intravenously (i.v.) and intratumorally (i.t.) to LLC-bearing female C57BL/6J mice. At various time points postadministration, the animals were sacrificed and transgene expression in the tumor and liver was determined quantitatively by the ONPG to ONP enzymatic conversion assay and qualitatively by X-gal staining.
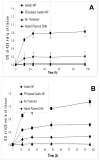
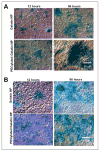
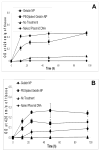
Results: Almost 100% of the pCMV-beta was encapsulated in gelatin and PEGylated gelatin nanoparticles (mean diameter 200 nm) at 0.5% (w/w) concentration. PEGylated gelatin nanoparticles efficiently transfected the LLC cells and the beta-galactosidase expression, as measured by the ONPG to ONP enzymatic conversion assay at 420 nm absorbance, increased starting from 12 h until 96 h post-transfection. The efficient expression of LLC cells was also evident by the X-gal staining method that shows blue color formation. The in vivo studies showed significant expression of beta-galactosidase in the tumor following administration of DNA-containing PEGylated gelatin nanoparticles to LLC-bearing mice by both i.v. and i.t. routes. Following i.v. administration of pCMV-beta in PEGylated gelatin nanoparticles, for instance, the absorbance at 420 nm per gram of tumor increased from 0.60 after 12 h to 0.85 after 96 h of transfection. After i.t. administration, the absorbance values increased from 0.90 after 12 h to almost 1.4 after 96 h.
Conclusions: The in vitro and in vivo results of this study clearly show that a long-circulating, biocompatible and biodegradable, DNA-encapsulating nanoparticulate system would be highly desirable for systemic delivery of genetic constructs to solid tumors.
Figures





Similar articles
-
Cellular interactions and in vitro DNA transfection studies with poly(ethylene glycol)-modified gelatin nanoparticles.J Pharm Sci. 2005 Jan;94(1):184-98. doi: 10.1002/jps.20216. J Pharm Sci. 2005. PMID: 15761942
-
Biodistribution and targeting potential of poly(ethylene glycol)-modified gelatin nanoparticles in subcutaneous murine tumor model.J Drug Target. 2004;12(9-10):585-91. doi: 10.1080/10611860400013451. J Drug Target. 2004. PMID: 15621684 Free PMC article.
-
Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery.Pharm Res. 2002 Jul;19(7):1061-7. doi: 10.1023/a:1016486910719. Pharm Res. 2002. PMID: 12180540
-
Non-condensing polymeric nanoparticles for targeted gene and siRNA delivery.Int J Pharm. 2012 May 1;427(1):21-34. doi: 10.1016/j.ijpharm.2011.05.036. Epub 2011 May 19. Int J Pharm. 2012. PMID: 21621597 Free PMC article. Review.
-
Modified gelatin nanoparticles for gene delivery.Int J Pharm. 2019 Jan 10;554:224-234. doi: 10.1016/j.ijpharm.2018.11.001. Epub 2018 Nov 5. Int J Pharm. 2019. PMID: 30408531 Review.
Cited by
-
Nanotechnology and CRISPR/Cas-Mediated Gene Therapy Strategies: Potential Role for Treating Genetic Disorders.Mol Biotechnol. 2024 Oct 24. doi: 10.1007/s12033-024-01301-8. Online ahead of print. Mol Biotechnol. 2024. PMID: 39446301 Review.
-
Protein-based nanoparticles for therapeutic nucleic acid delivery.Biomaterials. 2024 Mar;305:122464. doi: 10.1016/j.biomaterials.2023.122464. Epub 2024 Jan 2. Biomaterials. 2024. PMID: 38181574 Review.
-
Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment.Genes (Basel). 2023 Jun 28;14(7):1370. doi: 10.3390/genes14071370. Genes (Basel). 2023. PMID: 37510275 Free PMC article. Review.
-
Current Trends in Gelatin-Based Drug Delivery Systems.Pharmaceutics. 2023 May 15;15(5):1499. doi: 10.3390/pharmaceutics15051499. Pharmaceutics. 2023. PMID: 37242741 Free PMC article. Review.
-
Research progress of stimulus-responsive antibacterial materials for bone infection.Front Bioeng Biotechnol. 2022 Dec 23;10:1069932. doi: 10.3389/fbioe.2022.1069932. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36636700 Free PMC article. Review.
References
-
- von Eschenbach AC. A vision for the national cancer program in the United States. Nat Rev Cancer. 2004;4:820–828. - PubMed
-
- Baselga J. New horizons: gene therapy for cancer. Anticancer Drugs. 1999;10:S39–S42. - PubMed
-
- Huber BE. Gene therapy strategies for treating neoplastic diseases. Ann NY Acad Sci. 1994;716:6–11. - PubMed
-
- Roth JA, Christiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. - PubMed
-
- R.J. Mrsny. Tissue- and cell-specific targeting for the delivery of genetic information. In M.M. Amiji (ed.) Polymeric Gene Delivery: Principles and Applications. Chapter 2, CRC Press, LLC. Boca Raton, FL. 2004, pp. 5–27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

