Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling
- PMID: 15944737
- PMCID: PMC1173141
- DOI: 10.1038/sj.emboj.7600688
Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling
Abstract
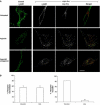
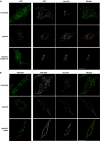
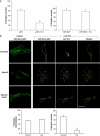
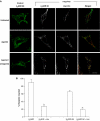
Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is well known to terminate cell signaling by sorting activated receptors to the MVB/lysosomal pathway. Here we identify a distinct role of Hrs in promoting rapid recycling of endocytosed signaling receptors to the plasma membrane. This function of Hrs is specific for receptors that recycle in a sequence-directed manner, in contrast to default recycling by bulk membrane flow, and is distinguishable in several ways from previously identified membrane-trafficking functions of Hrs/Vps27p. In particular, Hrs function in sequence-directed recycling does not require other mammalian Class E gene products involved in MVB/lysosomal sorting, nor is receptor ubiquitination required. Mutational studies suggest that the VHS domain of Hrs plays an important role in sequence-directed recycling. Disrupting Hrs-dependent recycling prevented functional resensitization of the beta(2)-adrenergic receptor, converting the temporal profile of cell signaling by this prototypic G protein-coupled receptor from sustained to transient. These studies identify a novel function of Hrs in a cargo-specific recycling mechanism, which is critical to controlling functional activity of the largest known family of signaling receptors.
Figures

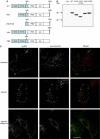
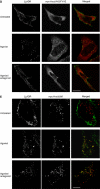
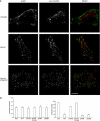
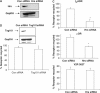
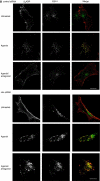
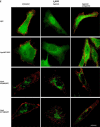
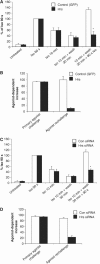
Similar articles
-
TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation.Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7626-31. doi: 10.1073/pnas.0932599100. Epub 2003 Jun 11. Proc Natl Acad Sci U S A. 2003. PMID: 12802020 Free PMC article.
-
Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking.Exp Cell Res. 2008 Feb 15;314(4):801-13. doi: 10.1016/j.yexcr.2007.10.014. Epub 2007 Nov 26. Exp Cell Res. 2008. PMID: 18031739
-
A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism.J Biol Chem. 2007 Feb 2;282(5):3095-104. doi: 10.1074/jbc.M605398200. Epub 2006 Nov 30. J Biol Chem. 2007. PMID: 17138565
-
The Hrs/STAM complex in the downregulation of receptor tyrosine kinases.J Biochem. 2005 Jan;137(1):1-8. doi: 10.1093/jb/mvi001. J Biochem. 2005. PMID: 15713877 Review.
-
Function of Hrs in endocytic trafficking and signalling.Biochem Soc Trans. 2001 Aug;29(Pt 4):472-5. doi: 10.1042/bst0290472. Biochem Soc Trans. 2001. PMID: 11498011 Review.
Cited by
-
Functional rewiring of G protein-coupled receptor signaling in human labor.Cell Rep. 2022 Sep 6;40(10):111318. doi: 10.1016/j.celrep.2022.111318. Cell Rep. 2022. PMID: 36070698 Free PMC article.
-
Regulation of epidermal growth factor receptor ubiquitination and trafficking by the USP8·STAM complex.J Biol Chem. 2010 Nov 5;285(45):34909-21. doi: 10.1074/jbc.M109.016287. Epub 2010 Aug 24. J Biol Chem. 2010. PMID: 20736164 Free PMC article.
-
The Myopic-Ubpy-Hrs nexus enables endosomal recycling of Frizzled.Mol Biol Cell. 2015 Sep 15;26(18):3329-42. doi: 10.1091/mbc.E15-02-0086. Epub 2015 Jul 29. Mol Biol Cell. 2015. PMID: 26224310 Free PMC article.
-
Rme-8 depletion perturbs Notch recycling and predisposes to pathogenic signaling.J Cell Biol. 2015 Jul 20;210(2):303-18. doi: 10.1083/jcb.201411001. Epub 2015 Jul 13. J Cell Biol. 2015. PMID: 26169355 Free PMC article.
-
Armadillo Repeat Containing 8alpha Binds to HRS and Promotes HRS Interaction with Ubiquitinated Proteins.Open Biochem J. 2010 Jan 13;4:1-8. doi: 10.2174/1874091X01004010001. Open Biochem J. 2010. PMID: 20224683 Free PMC article.
References
-
- Babst M, Odorizzi G, Estepa EJ, Emr SD (2000) Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248–258 - PubMed
-
- Bache KG, Raiborg C, Mehlum A, Stenmark H (2003) STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem 278: 12513–12521 - PubMed
-
- Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC (2002) The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat Cell Biol 4: 534–539 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

