Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles
- PMID: 15944223
- PMCID: PMC1182308
- DOI: 10.1091/mbc.e05-01-0020
Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles
Abstract
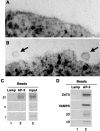
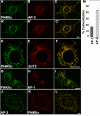
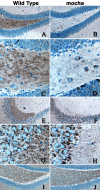
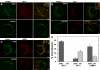
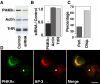
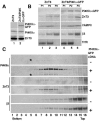
A membrane fraction enriched in vesicles containing the adaptor protein (AP) -3 cargo zinc transporter 3 was generated from PC12 cells and was used to identify new components of these organelles by mass spectrometry. Proteins prominently represented in the fraction included AP-3 subunits, synaptic vesicle proteins, and lysosomal proteins known to be sorted in an AP-3-dependent way or to interact genetically with AP-3. A protein enriched in this fraction was phosphatidylinositol-4-kinase type IIalpha (PI4KIIalpha). Biochemical, pharmacological, and morphological analyses supported the presence of PI4KIIalpha in AP-3-positive organelles. Furthermore, the subcellular localization of PI4KIIalpha was altered in cells from AP-3-deficient mocha mutant mice. The PI4KIIalpha normally present both in perinuclear and peripheral organelles was substantially decreased in the peripheral membranes of AP-3-deficient mocha fibroblasts. In addition, as is the case for other proteins sorted in an AP-3-dependent way, PI4KIIalpha content was strongly reduced in nerve terminals of mocha hippocampal mossy fibers. The functional relationship between AP-3 and PI4KIIalpha was further explored by PI4KIIalpha knockdown experiments. Reduction of the cellular content of PI4KIIalpha strongly decreased the punctate distribution of AP-3 observed in PC12 cells. These results indicate that PI4KIIalpha is present on AP-3 organelles where it regulates AP-3 function.
Figures


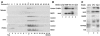

Similar articles
-
Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic.Mol Biol Cell. 2008 Apr;19(4):1415-26. doi: 10.1091/mbc.e07-12-1239. Epub 2008 Feb 6. Mol Biol Cell. 2008. PMID: 18256276 Free PMC article.
-
Calcyon, a mammalian specific NEEP21 family member, interacts with adaptor protein complex 3 (AP-3) and regulates targeting of AP-3 cargoes.J Neurochem. 2012 Oct;123(1):60-72. doi: 10.1111/j.1471-4159.2012.07814.x. Epub 2012 Aug 14. J Neurochem. 2012. PMID: 22650988 Free PMC article.
-
Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II alpha in neuronal and non-neuronal cells.J Biol Chem. 2009 Jan 16;284(3):1790-802. doi: 10.1074/jbc.M805991200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19010779 Free PMC article.
-
Neuronal and non-neuronal functions of the AP-3 sorting machinery.J Cell Sci. 2007 Feb 15;120(Pt 4):531-41. doi: 10.1242/jcs.03365. J Cell Sci. 2007. PMID: 17287392 Review.
-
What is the function of neuronal AP-3?Biol Cell. 2007 Jul;99(7):349-61. doi: 10.1042/BC20070029. Biol Cell. 2007. PMID: 17567262 Review.
Cited by
-
ORP1L mediated PI(4)P signaling at ER-lysosome-mitochondrion three-way contact contributes to mitochondrial division.Nat Commun. 2021 Sep 9;12(1):5354. doi: 10.1038/s41467-021-25621-4. Nat Commun. 2021. PMID: 34504082 Free PMC article.
-
Tetraspanin CD81 is required for Listeria monocytogenes invasion.Infect Immun. 2010 Jan;78(1):204-9. doi: 10.1128/IAI.00661-09. Epub 2009 Nov 9. Infect Immun. 2010. PMID: 19901060 Free PMC article.
-
An equal opportunity collaboration between lipid metabolism and proteins in the control of membrane trafficking in the trans-Golgi and endosomal systems.Curr Opin Cell Biol. 2019 Aug;59:58-72. doi: 10.1016/j.ceb.2019.03.012. Epub 2019 Apr 28. Curr Opin Cell Biol. 2019. PMID: 31039522 Free PMC article. Review.
-
Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila.Development. 2012 Aug;139(16):3040-50. doi: 10.1242/dev.077644. Epub 2012 Jul 12. Development. 2012. PMID: 22791894 Free PMC article.
-
BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles.Mol Biol Cell. 2007 Mar;18(3):768-80. doi: 10.1091/mbc.e06-12-1066. Epub 2006 Dec 20. Mol Biol Cell. 2007. PMID: 17182842 Free PMC article.
References
-
- Balla, A., Tuymetova, G., Barshishat, M., Geiszt, M., and Balla, T. (2002). Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 277, 20041–20050. - PubMed
-
- Barylko, B., Gerber, S. H., Binns, D. D., Grichine, N., Khvotchev, M., Sudhof, T. C., and Albanesi, J. P. (2001). A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 276, 7705–7708. - PubMed
-
- Benson, K. F., et al. (2003). Mutations associated with neutropenia in dogs and humans disrupt intracellular transport of neutrophil elastase. Nat. Genet. 35, 90–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

