Robust hepatitis C virus infection in vitro
- PMID: 15939869
- PMCID: PMC1166622
- DOI: 10.1073/pnas.0503596102
Robust hepatitis C virus infection in vitro
Abstract
The absence of a robust cell culture model of hepatitis C virus (HCV) infection has severely limited analysis of the HCV life cycle and the development of effective antivirals and vaccines. Here we report the establishment of a simple yet robust HCV cell culture infection system based on the HCV JFH-1 molecular clone and Huh-7-derived cell lines that allows the production of virus that can be efficiently propagated in tissue culture. This system provides a powerful tool for the analysis of host-virus interactions that should facilitate the discovery of antiviral drugs and vaccines for this important human pathogen.
Figures

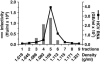
Comment in
-
Efficient hepatitis C virus cell culture system: what a difference the host cell makes.Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9739-40. doi: 10.1073/pnas.0504296102. Epub 2005 Jul 5. Proc Natl Acad Sci U S A. 2005. PMID: 15998731 Free PMC article. No abstract available.
Similar articles
-
Regulation of Hepatitis C Virus Infection by Cellular Retinoic Acid Binding Proteins through the Modulation of Lipid Droplet Abundance.J Virol. 2019 Apr 3;93(8):e02302-18. doi: 10.1128/JVI.02302-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728260 Free PMC article.
-
Production of infectious hepatitis C virus in tissue culture from a cloned viral genome.Nat Med. 2005 Jul;11(7):791-6. doi: 10.1038/nm1268. Epub 2005 Jun 12. Nat Med. 2005. PMID: 15951748 Free PMC article.
-
Tupaia CD81, SR-BI, claudin-1, and occludin support hepatitis C virus infection.J Virol. 2011 Mar;85(6):2793-802. doi: 10.1128/JVI.01818-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177818 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Mapping the scientific knowledge and approaches to defining and measuring hate crime, hate speech, and hate incidents: A systematic review.Campbell Syst Rev. 2024 Apr 28;20(2):e1397. doi: 10.1002/cl2.1397. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38686101 Free PMC article. Review.
Cited by
-
Hepatitis C virus and natural compounds: a new antiviral approach?Viruses. 2012 Oct 17;4(10):2197-217. doi: 10.3390/v4102197. Viruses. 2012. PMID: 23202460 Free PMC article. Review.
-
Expression of microRNA miR-122 facilitates an efficient replication in nonhepatic cells upon infection with hepatitis C virus.J Virol. 2012 Aug;86(15):7918-33. doi: 10.1128/JVI.00567-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593164 Free PMC article.
-
New hepatitis C virus drug discovery strategies and model systems.Expert Opin Drug Discov. 2012 Sep;7(9):849-59. doi: 10.1517/17460441.2012.711312. Epub 2012 Aug 4. Expert Opin Drug Discov. 2012. PMID: 22861052 Free PMC article. Review.
-
Hepatitis C virus and antiviral innate immunity: who wins at tug-of-war?World J Gastroenterol. 2015 Apr 7;21(13):3786-800. doi: 10.3748/wjg.v21.i13.3786. World J Gastroenterol. 2015. PMID: 25852264 Free PMC article. Review.
-
Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies.PLoS Pathog. 2013 Mar;9(3):e1003244. doi: 10.1371/journal.ppat.1003244. Epub 2013 Mar 21. PLoS Pathog. 2013. PMID: 23555257 Free PMC article.
References
-
- Hoofnagle, J. H. (2002) Hepatology 36, S21-S29. - PubMed
-
- Kanto, T., Hayashi, N., Takehara, T., Tatsumi, T., Kuzushita, N., Ito, A., Sasaki, Y., Kasahara, A. & Hori, M. (1999) J. Immunol. 162, 5584-5591. - PubMed
-
- Auffermann-Gretzinger, S., Keeffe, E. B. & Levy, S. (2001) Blood 97, 3171-3176. - PubMed
-
- Hiasa, Y., Horiike, N., Akbar, S. M., Saito, I., Miyamura, T., Matsuura, Y. & Onji, M. (1998) Biochem. Biophys. Res. Commun. 249, 90-95. - PubMed
-
- Alter, H. J. & Seeff, L. B. (2000) Semin. Liver Dis. 20, 17-35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

