Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP
- PMID: 15933714
- PMCID: PMC1173147
- DOI: 10.1038/sj.emboj.7600706
Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP
Abstract
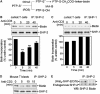
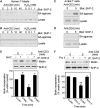
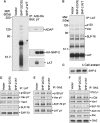
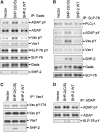
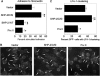
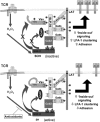
Receptor-stimulated generation of intracellular reactive oxygen species (ROS) modulates signal transduction, although the mechanism(s) is unclear. One potential basis is the reversible oxidation of the active site cysteine of protein tyrosine phosphatases (PTPs). Here, we show that activation of the antigen receptor of T cells (TCR), which induces production of ROS, induces transient inactivation of the SH2 domain-containing PTP, SHP-2, but not the homologous SHP-1. SHP-2 is recruited to the LAT-Gads-SLP-76 complex and directly regulates the phosphorylation of key signaling proteins Vav1 and ADAP. Furthermore, the association of ADAP with the adapter SLP-76 is regulated by SHP-2 in a redox-dependent manner. The data indicate that TCR-mediated ROS generation leads to SHP-2 oxidation, which promotes T-cell adhesion through effects on an SLP-76-dependent signaling pathway to integrin activation.
Figures

Similar articles
-
The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells.J Exp Med. 1998 May 4;187(9):1417-26. doi: 10.1084/jem.187.9.1417. J Exp Med. 1998. PMID: 9565634 Free PMC article.
-
Hematopoietic cell phosphatase, SHP-1, is constitutively associated with the SH2 domain-containing leukocyte protein, SLP-76, in B cells.J Exp Med. 1996 Aug 1;184(2):457-63. doi: 10.1084/jem.184.2.457. J Exp Med. 1996. PMID: 8760799 Free PMC article.
-
Positive and negative regulation by SLP-76/ADAP and Pyk2 of chemokine-stimulated T-lymphocyte adhesion mediated by integrin α4β1.Mol Biol Cell. 2015 Sep 15;26(18):3215-28. doi: 10.1091/mbc.E14-07-1246. Epub 2015 Jul 22. Mol Biol Cell. 2015. PMID: 26202465 Free PMC article.
-
ADAP-ting TCR signaling to integrins.Sci STKE. 2002 Apr 9;2002(127):re3. doi: 10.1126/stke.2002.127.re3. Sci STKE. 2002. PMID: 11943877 Review.
-
Reactive oxygen species as mediators of cell adhesion.Ital J Biochem. 2003 Mar;52(1):28-32. Ital J Biochem. 2003. PMID: 12833635 Review.
Cited by
-
Reactive Oxygen Species: Angels and Demons in the Life of a Neuron.NeuroSci. 2022 Mar 16;3(1):130-145. doi: 10.3390/neurosci3010011. eCollection 2022 Mar. NeuroSci. 2022. PMID: 39484669 Free PMC article. Review.
-
SHP-1 Acts as a Key Regulator of Alloresponses by Modulating LFA-1-Mediated Adhesion in Primary Murine T Cells.Mol Cell Biol. 2016 Nov 28;36(24):3113-3127. doi: 10.1128/MCB.00294-16. Print 2016 Dec 15. Mol Cell Biol. 2016. PMID: 27697866 Free PMC article.
-
Methods to monitor classical protein-tyrosine phosphatase oxidation.FEBS J. 2013 Jan;280(2):459-75. doi: 10.1111/j.1742-4658.2012.08626.x. Epub 2012 May 30. FEBS J. 2013. PMID: 22577968 Free PMC article. Review.
-
Selective activation of oxidized PTP1B by the thioredoxin system modulates PDGF-β receptor tyrosine kinase signaling.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13398-403. doi: 10.1073/pnas.1302891110. Epub 2013 Jul 30. Proc Natl Acad Sci U S A. 2013. PMID: 23901112 Free PMC article.
-
Peroxiredoxin II is an antioxidant enzyme that negatively regulates collagen-stimulated platelet function.J Biol Chem. 2015 May 1;290(18):11432-42. doi: 10.1074/jbc.M115.644260. Epub 2015 Mar 23. J Biol Chem. 2015. PMID: 25802339 Free PMC article.
References
-
- Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB (1999) Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem 274: 34543–34546 - PubMed
-
- Boerth NJ, Judd BA, Koretzky GA (2000) Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J Biol Chem 275: 5143–5152 - PubMed
-
- Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G (2001) Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem 276: 33478–33487 - PubMed
-
- Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ III, Charrier V, Parsonage D (1999) Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 38: 15407–15416 - PubMed
-
- Dechert U, Adam M, Harder KW, Clark-Lewis I, Jirik F (1994) Characterization of protein tyrosine phosphatase SH-PTP2. Study of phosphopeptide substrates and possible regulatory role of SH2 domains. J Biol Chem 269: 5602–5611 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

