Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast
- PMID: 15930123
- PMCID: PMC1182314
- DOI: 10.1091/mbc.e05-04-0275
Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast
Abstract
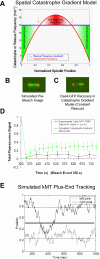
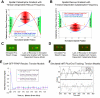
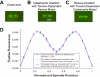
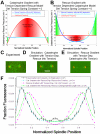
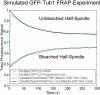
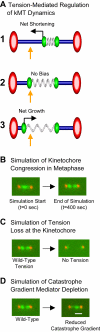
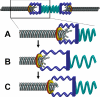
During metaphase in budding yeast mitosis, sister kinetochores are tethered to opposite poles and separated, stretching their intervening chromatin, by singly attached kinetochore microtubules (kMTs). Kinetochore movements are coupled to single microtubule plus-end polymerization/depolymerization at kinetochore attachment sites. Here, we use computer modeling to test possible mechanisms controlling chromosome alignment during yeast metaphase by simulating experiments that determine the 1) mean positions of kinetochore Cse4-GFP, 2) extent of oscillation of kinetochores during metaphase as measured by fluorescence recovery after photobleaching (FRAP) of kinetochore Cse4-GFP, 3) dynamics of kMTs as measured by FRAP of GFP-tubulin, and 4) mean positions of unreplicated chromosome kinetochores that lack pulling forces from a sister kinetochore. We rule out a number of possible models and find the best fit between theory and experiment when it is assumed that kinetochores sense both a spatial gradient that suppresses kMT catastrophe near the poles and attachment site tension that promotes kMT rescue at higher amounts of chromatin stretch.
Figures
Similar articles
-
Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase.Curr Biol. 2004 Nov 9;14(21):1962-7. doi: 10.1016/j.cub.2004.09.086. Curr Biol. 2004. PMID: 15530400
-
Mechanisms of microtubule-based kinetochore positioning in the yeast metaphase spindle.Biophys J. 2003 Jun;84(6):3529-46. doi: 10.1016/S0006-3495(03)75087-5. Biophys J. 2003. PMID: 12770865 Free PMC article.
-
Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase.Curr Biol. 2006 Aug 8;16(15):1489-501. doi: 10.1016/j.cub.2006.06.063. Curr Biol. 2006. PMID: 16890524 Free PMC article.
-
Kinetochore-microtubule coupling mechanisms mediated by the Ska1 complex and Cdt1.Essays Biochem. 2020 Sep 4;64(2):337-347. doi: 10.1042/EBC20190075. Essays Biochem. 2020. PMID: 32844209 Free PMC article. Review.
-
Structures and functions of yeast kinetochore complexes.Annu Rev Biochem. 2007;76:563-91. doi: 10.1146/annurev.biochem.76.052705.160607. Annu Rev Biochem. 2007. PMID: 17362199 Review.
Cited by
-
Uncovering chromatin's contribution to the mitotic spindle: Applications of computational and polymer models.Biochimie. 2010 Dec;92(12):1741-8. doi: 10.1016/j.biochi.2010.06.014. Epub 2010 Jun 25. Biochimie. 2010. PMID: 20600566 Free PMC article.
-
Metaphase kinetochore movements are regulated by kinesin-8 motors and microtubule dynamic instability.Mol Biol Cell. 2018 Jun 1;29(11):1332-1345. doi: 10.1091/mbc.E17-11-0667. Epub 2018 Apr 5. Mol Biol Cell. 2018. PMID: 29851559 Free PMC article.
-
Microtubule assembly dynamics: new insights at the nanoscale.Curr Opin Cell Biol. 2008 Feb;20(1):64-70. doi: 10.1016/j.ceb.2007.12.003. Curr Opin Cell Biol. 2008. PMID: 18243676 Free PMC article. Review.
-
Kinesin-8 molecular motors: putting the brakes on chromosome oscillations.Trends Cell Biol. 2008 Jul;18(7):307-10. doi: 10.1016/j.tcb.2008.05.003. Epub 2008 May 29. Trends Cell Biol. 2008. PMID: 18513970 Free PMC article. Review.
-
A TOGL domain specifically targets yeast CLASP to kinetochores to stabilize kinetochore microtubules.J Cell Biol. 2014 May 26;205(4):555-71. doi: 10.1083/jcb.201310018. J Cell Biol. 2014. PMID: 24862575 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

