Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5
- PMID: 15923619
- PMCID: PMC1140585
- DOI: 10.1128/MCB.25.12.5022-5030.2005
Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5
Abstract
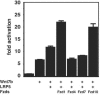
Wnt7b is a Wnt ligand that has been demonstrated to play critical roles in several developmental processes, including lung airway and vascular development and chorion-allantois fusion during placental development. Wnt signaling involves the binding of Wnt ligands to cell surface receptors of the frizzled family and coreceptors of the LRP5/6 family. However, little is known of the ligand-receptor specificity exhibited by different Wnts, Fzds, and LRPs in Wnt signaling. Expression analysis of Fzds and LRP5/6 in the developing lung and vasculature showed that Fzd1, -4, -7, and -10 and LRP5/6 are expressed in tissue-specific patterns during lung development. Fzd1, -4, and -7 are expressed primarily in the developing lung mesenchyme, and Fzd10 is expressed in airway epithelium. LRP5 and LRP6 are expressed in airway epithelium during lung development, whereas LRP5 but not LRP6 expression is observed in the muscular component of large blood vessels, including the aorta. Cell transfection studies demonstrate that Wnt7b can activate the canonical Wnt pathway but not the noncanonical Wnt pathway in a cell-specific manner. Biochemical analysis demonstrates that Wnt7b can bind to Fzd1 and -10 on the cell surface and cooperatively activate canonical Wnt signaling with these receptors in the presence of LRP5. Together, these data demonstrate that Wnt7b signals through Fzd1 and -10 and LRP5 and implicate these Wnt coreceptors in the regulation of lung airway and vascular development.
Figures



Similar articles
-
β-Catenin-dependent pathway activation by both promiscuous "canonical" WNT3a-, and specific "noncanonical" WNT4- and WNT5a-FZD receptor combinations with strong differences in LRP5 and LRP6 dependency.Cell Signal. 2014 Feb;26(2):260-7. doi: 10.1016/j.cellsig.2013.11.021. Epub 2013 Nov 21. Cell Signal. 2014. PMID: 24269653
-
Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling.J Biol Chem. 2005 May 20;280(20):19883-7. doi: 10.1074/jbc.M413274200. Epub 2005 Mar 18. J Biol Chem. 2005. PMID: 15778503
-
Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors.BMC Cell Biol. 2003 May 2;4:4. doi: 10.1186/1471-2121-4-4. BMC Cell Biol. 2003. PMID: 12729465 Free PMC article.
-
LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way.Development. 2004 Apr;131(8):1663-77. doi: 10.1242/dev.01117. Development. 2004. PMID: 15084453 Review.
-
WNT signaling in stem cell biology and regenerative medicine.Curr Drug Targets. 2008 Jul;9(7):565-70. doi: 10.2174/138945008784911750. Curr Drug Targets. 2008. PMID: 18673242 Review.
Cited by
-
Wnt ligands signal in a cooperative manner to promote foregut organogenesis.Proc Natl Acad Sci U S A. 2012 Sep 18;109(38):15348-53. doi: 10.1073/pnas.1201583109. Epub 2012 Sep 4. Proc Natl Acad Sci U S A. 2012. PMID: 22949635 Free PMC article.
-
Airway epithelial cell isolation techniques affect DNA methylation profiles with consequences for analysis of asthma related perturbations to DNA methylation.Sci Rep. 2019 Oct 8;9(1):14409. doi: 10.1038/s41598-019-50873-y. Sci Rep. 2019. PMID: 31595000 Free PMC article.
-
The role of cancer stem cells in neoplasia of the lung: past, present and future.Clin Transl Oncol. 2008 Nov;10(11):719-25. doi: 10.1007/s12094-008-0278-6. Clin Transl Oncol. 2008. PMID: 19015068 Review.
-
Wnt/Frizzled family members mediate olfactory sensory neuron axon extension.J Comp Neurol. 2008 Nov 20;511(3):301-17. doi: 10.1002/cne.21834. J Comp Neurol. 2008. PMID: 18803244 Free PMC article.
-
Airway response to acute mechanical stress in a human bronchial model of stretch.Crit Care. 2011;15(5):R208. doi: 10.1186/cc10443. Epub 2011 Sep 13. Crit Care. 2011. PMID: 21914176 Free PMC article.
References
-
- Bafico, A., A. Gazit, S. S. Wu-Morgan, A. Yaniv, and S. A. Aaronson. 1998. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene 16:2819-2825. - PubMed
-
- Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. - PubMed
-
- Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang, J. P. Macke, D. Andrew, J. Nathans, and R. Nusse. 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382:225-230. - PubMed
-
- Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
