STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3
- PMID: 15919823
- PMCID: PMC1149424
- DOI: 10.1073/pnas.0501643102
STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3
Abstract
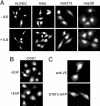
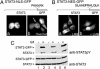
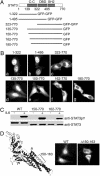
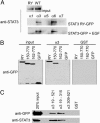
Signal transducer and activator of transcription (STAT)3 is a member of a family of DNA-binding factors that function to induce expression of responsive genes. STAT3 can act as an oncogene, and its function has been shown to be critical for cellular transformation by a number of oncogenic tyrosine kinases. The role of STAT3 as a DNA-binding transcription factor naturally depends on its ability to gain entrance to the nucleus. In this study, we provide evidence that STAT3 is distinct from previously characterized STAT molecules in that it dynamically shuttles between cytoplasmic and nuclear compartments and maintains prominent nuclear presence. Although tyrosine phosphorylation is required for STAT3 to bind to specific DNA target sites, nuclear import takes place constitutively and independently of tyrosine phosphorylation. We identify a region within the coiled-coil domain of the STAT3 molecule that is necessary for nuclear import and demonstrate that this region is critical for its recognition by specific import carrier importin-alpha3. RNA interference studies were used to verify the role and specificity of importin-alpha3 in STAT3 nuclear translocation. These results distinguish STAT3 cellular localization from other STAT molecules and identify a feature that may be targeted in the clinical intervention of STAT3-dependent neoplasia.
Figures

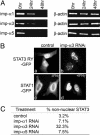
Similar articles
-
Extracellular signal-dependent nuclear import of STAT3 is mediated by various importin alphas.Biochem Biophys Res Commun. 2005 May 13;330(3):880-6. doi: 10.1016/j.bbrc.2005.03.063. Biochem Biophys Res Commun. 2005. PMID: 15809078
-
Dissecting functions of the N-terminal domain and GAS-site recognition in STAT3 nuclear trafficking.Cell Signal. 2016 Aug;28(8):810-25. doi: 10.1016/j.cellsig.2016.03.011. Epub 2016 Mar 31. Cell Signal. 2016. PMID: 27040695
-
Nuclear retention of STAT3 through the coiled-coil domain regulates its activity.Biochem Biophys Res Commun. 2005 Oct 21;336(2):617-24. doi: 10.1016/j.bbrc.2005.08.145. Biochem Biophys Res Commun. 2005. PMID: 16140268
-
The ins and outs of STAT1 nuclear transport.Sci STKE. 2003 Aug 12;2003(195):RE13. doi: 10.1126/stke.2003.195.re13. Sci STKE. 2003. PMID: 12915721 Review.
-
STATs get their move on.JAKSTAT. 2013 Oct 1;2(4):e27080. doi: 10.4161/jkst.27080. Epub 2013 Nov 13. JAKSTAT. 2013. PMID: 24470978 Free PMC article. Review.
Cited by
-
Granulin, a novel STAT3-interacting protein, enhances STAT3 transcriptional function and correlates with poorer prognosis in breast cancer.Genes Cancer. 2015 Mar;6(3-4):153-68. doi: 10.18632/genesandcancer.58. Genes Cancer. 2015. PMID: 26000098 Free PMC article.
-
Chaperoning STAT3/5 by Heat Shock Proteins: Interest of Their Targeting in Cancer Therapy.Cancers (Basel). 2019 Dec 19;12(1):21. doi: 10.3390/cancers12010021. Cancers (Basel). 2019. PMID: 31861612 Free PMC article. Review.
-
The role of STATs in lung carcinogenesis: an emerging target for novel therapeutics.J Mol Med (Berl). 2007 May;85(5):427-36. doi: 10.1007/s00109-006-0152-3. Epub 2007 Jan 10. J Mol Med (Berl). 2007. PMID: 17216202 Review.
-
Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury.Cell Cycle. 2019 Nov;18(21):3010-3029. doi: 10.1080/15384101.2019.1667189. Epub 2019 Sep 18. Cell Cycle. 2019. PMID: 31530090 Free PMC article.
-
Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling.Mol Biol Cell. 2010 Jan 15;21(2):311-22. doi: 10.1091/mbc.e09-05-0437. Epub 2009 Nov 25. Mol Biol Cell. 2010. PMID: 19940019 Free PMC article.
References
-
- Akira, S. (1999) Stem Cells 17, 138-146. - PubMed
-
- Bromberg, J. & Darnell, J. E., Jr. (2000) Oncogene 19, 2468-2473. - PubMed
-
- Darnell, J. E., Jr. (1997) Science 277, 1630-1635. - PubMed
-
- Ihle, J. N. (2001) Curr. Opin. Cell Biol. 13, 211-217. - PubMed
-
- Davis, L. I. (1995) Annu. Rev. Biochem. 64, 865-896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

