Zebrafish hairy/enhancer of split protein links FGF signaling to cyclic gene expression in the periodic segmentation of somites
- PMID: 15905406
- PMCID: PMC1132002
- DOI: 10.1101/gad.1291205
Zebrafish hairy/enhancer of split protein links FGF signaling to cyclic gene expression in the periodic segmentation of somites
Abstract
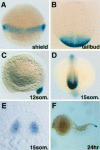
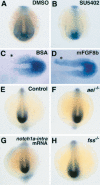
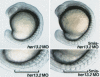
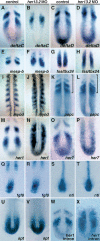
Notch and fibroblast growth factor (FGF) signaling pathways have been implicated in the establishment of proper periodicity of vertebrate somites. Here, we show evidence that a Hes6-related hairy/Enhancer of split-related gene, her13.2, links FGF signaling to the Notch-regulated oscillation machinery in zebrafish. Expression of her13.2 is induced by FGF-soaked beads and decreased by an FGF signaling inhibitor. her13.2 is required for periodic repression of the Notch-regulated genes her1 and her7, and for proper somite segmentation. Furthermore, Her13.2 augments autorepression of her1 in association with Her1 protein. Therefore, FGF signaling appears to maintain the oscillation machinery by supplying a binding partner, Her13.2, for Her1.
Figures
Similar articles
-
Posterior-anterior gradient of zebrafish hes6 expression in the presomitic mesoderm is established by the combinatorial functions of the downstream enhancer and 3'UTR.Dev Biol. 2016 Jan 15;409(2):543-54. doi: 10.1016/j.ydbio.2015.11.010. Epub 2015 Nov 17. Dev Biol. 2016. PMID: 26596999
-
Two linked hairy/Enhancer of split-related zebrafish genes, her1 and her7, function together to refine alternating somite boundaries.Development. 2002 Aug;129(15):3693-704. doi: 10.1242/dev.129.15.3693. Development. 2002. PMID: 12117818
-
Emergence of traveling waves in the zebrafish segmentation clock.Development. 2010 May;137(10):1595-9. doi: 10.1242/dev.046888. Epub 2010 Apr 14. Development. 2010. PMID: 20392739
-
Oscillatory links of Fgf signaling and Hes7 in the segmentation clock.Curr Opin Genet Dev. 2013 Aug;23(4):484-90. doi: 10.1016/j.gde.2013.02.005. Epub 2013 Mar 4. Curr Opin Genet Dev. 2013. PMID: 23465881 Review.
-
Oscillatory gene expression and somitogenesis.Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):629-41. doi: 10.1002/wdev.46. Epub 2012 Mar 22. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799565 Review.
Cited by
-
In vivo Functional Genomics for Undiagnosed Patients: The Impact of Small GTPases Signaling Dysregulation at Pan-Embryo Developmental Scale.Front Cell Dev Biol. 2021 May 25;9:642235. doi: 10.3389/fcell.2021.642235. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34124035 Free PMC article. Review.
-
Geminin Orchestrates Somite Formation by Regulating Fgf8 and Notch Signaling.Biomed Res Int. 2018 Jun 7;2018:6543196. doi: 10.1155/2018/6543196. eCollection 2018. Biomed Res Int. 2018. PMID: 29984243 Free PMC article.
-
Signalling dynamics in vertebrate segmentation.Nat Rev Mol Cell Biol. 2014 Nov;15(11):709-21. doi: 10.1038/nrm3891. Nat Rev Mol Cell Biol. 2014. PMID: 25335437 Review.
-
The segmentation clock mechanism moves up a notch.Trends Cell Biol. 2010 Oct;20(10):593-600. doi: 10.1016/j.tcb.2010.07.001. Epub 2010 Aug 18. Trends Cell Biol. 2010. PMID: 20724159 Free PMC article. Review.
-
Effects of microRNA-305 knockdown on brain gene expression associated with division of labor in honey bee colonies (Apis mellifera).J Exp Biol. 2024 Apr 15;227(8):jeb246785. doi: 10.1242/jeb.246785. Epub 2024 Apr 30. J Exp Biol. 2024. PMID: 38517067 Free PMC article.
References
-
- Aulehla A., Wehrle, C., Brand-Saberi, B., Kemler, R., Gossler, A., Kanzler, B., and Herrmann, B.G. 2003. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 4: 395–406. - PubMed
-
- Bae S., Bessho, Y., Hojo, M., and Kageyama, R. 2000. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development 127: 2933–2943. - PubMed
-
- Bessho Y. and Kageyama, R. 2003. Oscillations, clocks and segmentation. Curr. Opin. Genet. Dev. 13: 379–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
