Systematic review to determine whether participation in a trial influences outcome
- PMID: 15905256
- PMCID: PMC558011
- DOI: 10.1136/bmj.330.7501.1175
Systematic review to determine whether participation in a trial influences outcome
Abstract
Objective: To systematically compare the outcomes of participants in randomised controlled trials (RCTs) with those in comparable non-participants who received the same or similar treatment.
Data sources: Bibliographic databases, reference lists from eligible articles, medical journals, and study authors.
Review methods: RCTs and cohort studies that evaluated the clinical outcomes of participants in RCTs and comparable non-participants who received the same or similar treatment.
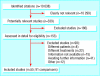
Results: Five RCTs (six comparisons) and 50 cohort studies (85 comparisons) provided data on 31,140 patients treated in RCTs and 20,380 comparable patients treated outside RCTs. In the five RCTs, in which patients were given the option of participating or not, the comparisons provided limited information because of small sample sizes (a total of 412 patients) and the nature of the questions considered. 73 dichotomous outcomes were compared, of which 59 reported no statistically significant differences. For patients treated within RCTs, 10 comparisons reported significantly better outcomes and four reported significantly worse outcomes. Significantly heterogeneity was found (I2 = 89%) among the comparisons of 73 dichotomous outcomes; none of our a priori explanatory factors helped explain this heterogeneity. The 18 comparisons of continuous outcomes showed no significant differences in heterogeneity (I2 = 0%). The overall pooled estimate for continuous outcomes of the effect of participating in an RCT was not significant (standardised mean difference 0.01, 95% confidence interval -0.10 to 0.12).
Conclusion: No strong evidence was found of a harmful or beneficial effect of participating in RCTs compared with receiving the same or similar treatment outside such trials.
Figures




Comment in
-
Participants in research.BMJ. 2005 May 21;330(7501):1164. doi: 10.1136/bmj.330.7501.1164. BMJ. 2005. PMID: 15905233 Free PMC article. No abstract available.
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Outcomes of patients who participate in randomised controlled trials compared to similar patients receiving similar interventions who do not participate.Cochrane Database Syst Rev. 2007 Apr 18;(2):MR000009. doi: 10.1002/14651858.MR000009.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2008 Jul 16;(3):MR000009. doi: 10.1002/14651858.MR000009.pub4 PMID: 17443630 Updated. Review.
-
Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate.Cochrane Database Syst Rev. 2008 Jul 16;2008(3):MR000009. doi: 10.1002/14651858.MR000009.pub4. Cochrane Database Syst Rev. 2008. PMID: 18677782 Free PMC article. Review.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) acute day hospital versus admission; (2) vocational rehabilitation; (3) day hospital versus outpatient care.Health Technol Assess. 2001;5(21):1-75. doi: 10.3310/hta5210. Health Technol Assess. 2001. PMID: 11532238 Review.
-
Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):MR000034. doi: 10.1002/14651858.MR000034.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:MR000034. doi: 10.1002/14651858.MR000034.pub3 PMID: 24782322 Free PMC article. Updated. Review.
Cited by
-
Outcomes of Patients with Bacillus Calmette-Guérin (BCG)-Unresponsive Non-Muscle Invasive Bladder Cancer as Defined by the U.S. Food and Drug Administration.Bladder Cancer. 2022 Sep 15;8(3):303-314. doi: 10.3233/BLC-211657. eCollection 2022. Bladder Cancer. 2022. PMID: 38993682 Free PMC article.
-
When are clinical trials beneficial for study patients and future patients? A factorial vignette-based survey of institutional review board members.BMJ Open. 2016 Sep 28;6(9):e011150. doi: 10.1136/bmjopen-2016-011150. BMJ Open. 2016. PMID: 27683511 Free PMC article.
-
Use of a motivational interviewing-informed strategy in group orientations to improve retention and intervention attendance in a randomized controlled trial.Health Educ Res. 2016 Dec;31(6):729-737. doi: 10.1093/her/cyw048. Epub 2016 Oct 10. Health Educ Res. 2016. PMID: 27923862 Free PMC article.
-
An Evaluation of IMPACT for the Treatment of Late-Life Depression in a Public Mental Health System.J Behav Health Serv Res. 2015 Jul;42(3):334-45. doi: 10.1007/s11414-013-9373-8. J Behav Health Serv Res. 2015. PMID: 24177923
-
Chemotherapy-related hospitalization among community cancer center patients.Oncologist. 2011;16(3):378-87. doi: 10.1634/theoncologist.2010-0354. Epub 2011 Feb 24. Oncologist. 2011. PMID: 21349949 Free PMC article.
References
-
- Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?” Lancet 2005;365: 82-93. - PubMed
-
- Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.” J Clin Epidemiol 2001;54: 217-24. - PubMed
-
- The Emergency Care Research Institute 2002. Patients' reasons for participation in clinical trials and effect of trial participation on patient outcomes. www.ecri.org/Patient_Information/Patient_Reference_Guide/evidence.pdf (accessed Nov 2004).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
