Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein
- PMID: 15901789
- PMCID: PMC6724851
- DOI: 10.1523/JNEUROSCI.1427-05.2005
Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein
Abstract
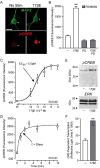
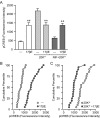
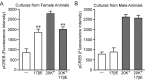
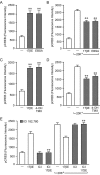
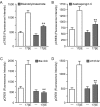
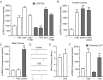
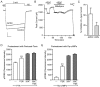
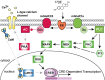
In addition to mediating sexual maturation and reproduction through stimulation of classical intracellular receptors that bind DNA and regulate gene expression, estradiol is also thought to influence various brain functions by acting on receptors localized to the neuronal membrane surface. Many intracellular signaling pathways and modulatory proteins are affected by estradiol via this unconventional route, including regulation of the transcription factor cAMP response element-binding protein (CREB). However, the mechanisms by which estradiol acts at the membrane surface are poorly understood. Because both estradiol and CREB have been implicated in regulating learning and memory, we characterized the effects of estradiol on this transcription factor in cultured rat hippocampal neurons. Within minutes of administration, estradiol triggered mitogen-activated protein kinase (MAPK)-dependent CREB phosphorylation in unstimulated neurons. Furthermore, after brief depolarization, estradiol attenuated L-type calcium channel-mediated CREB phosphorylation. Thus, estradiol exhibited both positive and negative influences on CREB activity. These effects of estradiol were sex specific and traced to membrane-localized estrogen receptors that stimulated group I and II metabotropic glutamate receptor (mGluR) signaling. Activation of estrogen receptor alpha (ERalpha) led to mGluR1a signaling, triggering CREB phosphorylation through phospholipase C regulation of MAPK. In addition, estradiol stimulation of ERalpha or ERbeta triggered mGluR2/3 signaling, decreasing L-type calcium channel-mediated CREB phosphorylation. These results not only characterize estradiol regulation of CREB but also provide two putative signaling mechanisms that may account for many of the unexplained observations regarding the influence of estradiol on nervous system function.
Figures


Similar articles
-
Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons.Neuroscience. 2010 Nov 10;170(4):1045-55. doi: 10.1016/j.neuroscience.2010.08.012. Epub 2010 Aug 13. Neuroscience. 2010. PMID: 20709161 Free PMC article.
-
Group I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to cyclic AMP response element binding protein (CREB) through a common Ca2+ - and protein kinase C-dependent pathway.J Neurochem. 2005 Apr;93(1):232-45. doi: 10.1111/j.1471-4159.2005.03012.x. J Neurochem. 2005. PMID: 15773922
-
Glutamate cascade to cAMP response element-binding protein phosphorylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors.Mol Pharmacol. 2002 Sep;62(3):473-84. doi: 10.1124/mol.62.3.473. Mol Pharmacol. 2002. PMID: 12181423
-
Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex.J Neurosci. 2002 May 1;22(9):3434-44. doi: 10.1523/JNEUROSCI.22-09-03434.2002. J Neurosci. 2002. PMID: 11978820 Free PMC article.
-
Nuclear calcium signaling.Adv Exp Med Biol. 2012;970:377-405. doi: 10.1007/978-3-7091-0932-8_17. Adv Exp Med Biol. 2012. PMID: 22351065 Review.
Cited by
-
Multiple estrogen receptor subtypes influence ingestive behavior in female rodents.Physiol Behav. 2015 Dec 1;152(Pt B):431-7. doi: 10.1016/j.physbeh.2015.05.032. Epub 2015 May 31. Physiol Behav. 2015. PMID: 26037634 Free PMC article. Review.
-
Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model.Elife. 2016 Apr 15;5:e12917. doi: 10.7554/eLife.12917. Elife. 2016. PMID: 27083045 Free PMC article.
-
Acute neuroestrogen blockade attenuates song-induced immediate early gene expression in auditory regions of male and female zebra finches.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Jan;206(1):15-31. doi: 10.1007/s00359-019-01382-w. Epub 2019 Nov 28. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31781892 Free PMC article.
-
Infralimbic Estradiol Enhances Neuronal Excitability and Facilitates Extinction of Cocaine Seeking in Female Rats via a BDNF/TrkB Mechanism.Front Behav Neurosci. 2019 Jul 31;13:168. doi: 10.3389/fnbeh.2019.00168. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31417375 Free PMC article.
-
Estrogen Receptor-A in Medial Preoptic Area Contributes to Sex Difference of Mice in Response to Sevoflurane Anesthesia.Neurosci Bull. 2022 Jul;38(7):703-719. doi: 10.1007/s12264-022-00825-w. Epub 2022 Feb 17. Neurosci Bull. 2022. PMID: 35175557 Free PMC article.
References
-
- Abraham IM, Todman MG, Korach KS, Herbison AE (2004) Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology 145: 3055-3061. - PubMed
-
- Aloisi AM (2003) Gonadal hormones and sex differences in pain reactivity. Clin J Pain 19: 168-174. - PubMed
-
- Arnold AP, Breedlove SM (1985) Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav 19: 469-498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
