An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus
- PMID: 15892035
- PMCID: PMC7167940
- DOI: 10.1002/path.1769
An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus
Abstract
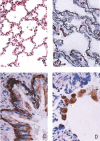
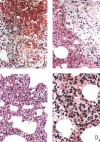
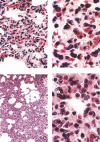
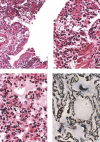
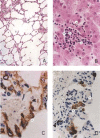
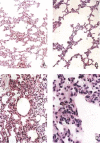
A new SARS animal model was established by inoculating SARS coronavirus (SARS-CoV) into rhesus macaques (Macaca mulatta) through the nasal cavity. Pathological pulmonary changes were successively detected on days 5-60 after virus inoculation. All eight animals showed a transient fever 2-3 days after inoculation. Immunological, molecular biological, and pathological studies support the establishment of this SARS animal model. Firstly, SARS-CoV-specific IgGs were detected in the sera of macaques from 11 to 60 days after inoculation. Secondly, SARS-CoV RNA could be detected in pharyngeal swab samples using nested RT-PCR in all infected animals from 5 days after virus inoculation. Finally, histopathological changes of interstitial pneumonia were found in the lungs during the 60 days after viral inoculation: these changes were less marked at later time points, indicating that an active healing process together with resolution of an acute inflammatory response was taking place in these animals. This animal model should provide insight into the mechanisms of SARS-CoV-related pulmonary disease and greatly facilitate the development of vaccines and therapeutics against SARS.
Copyright 2005 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures
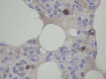

Similar articles
-
Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection.JCI Insight. 2019 Feb 21;4(4):e123158. doi: 10.1172/jci.insight.123158. eCollection 2019 Feb 21. JCI Insight. 2019. PMID: 30830861 Free PMC article.
-
Intratracheal inoculation of severe acute respiratory syndrome coronavirus in monkeys Macaca rhesus.Acta Virol. 2007;51(3):171-7. Acta Virol. 2007. PMID: 18076307
-
Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome.Lancet. 2003 Jul 26;362(9380):263-70. doi: 10.1016/S0140-6736(03)13967-0. Lancet. 2003. PMID: 12892955 Free PMC article.
-
SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium.Virus Res. 2008 Apr;133(1):33-44. doi: 10.1016/j.virusres.2007.03.013. Epub 2007 Apr 23. Virus Res. 2008. PMID: 17451829 Free PMC article. Review.
-
The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis.Antiviral Res. 2017 Jul;143:142-150. doi: 10.1016/j.antiviral.2017.03.022. Epub 2017 Apr 5. Antiviral Res. 2017. PMID: 28390872 Free PMC article. Review.
Cited by
-
miRNAs: The Key Regulator of COVID-19 Disease.Int J Cell Biol. 2022 Oct 29;2022:1645366. doi: 10.1155/2022/1645366. eCollection 2022. Int J Cell Biol. 2022. PMID: 36345541 Free PMC article. Review.
-
In silico design of refined ferritin-SARS-CoV-2 glyco-RBD nanoparticle vaccine.Front Mol Biosci. 2022 Sep 6;9:976490. doi: 10.3389/fmolb.2022.976490. eCollection 2022. Front Mol Biosci. 2022. PMID: 36148012 Free PMC article.
-
Vaccines platforms and COVID-19: what you need to know.Trop Dis Travel Med Vaccines. 2022 Aug 15;8(1):20. doi: 10.1186/s40794-022-00176-4. Trop Dis Travel Med Vaccines. 2022. PMID: 35965345 Free PMC article. Review.
-
Review of selected animal models for respiratory coronavirus infection and its application in drug research.J Med Virol. 2022 Jul;94(7):3032-3042. doi: 10.1002/jmv.27718. Epub 2022 Mar 24. J Med Virol. 2022. PMID: 35285034 Free PMC article. Review.
-
SARS-CoV-2 and wastewater: What does it mean for non-human primates?Am J Primatol. 2022 May;84(4-5):e23340. doi: 10.1002/ajp.23340. Epub 2021 Oct 18. Am J Primatol. 2022. PMID: 34662463 Free PMC article.
References
-
- Marra MA, Jones SJ, Astell CR, et al. The genome sequence of the SARS‐associated coronavirus. Science 2003; 300: 1399–1404. - PubMed
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–1976. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1953–1966. - PubMed
-
- Calza L, Manfredi R, Verucchi G, et al. SARS: a new emergency in the world health. Recent Prog Med 2003; 94: 284–294. - PubMed
-
- Chiu RW, Chim SS, Lo YM. Molecular epidemiology of SARS—from Amoy Gardens to Taiwan. N Engl J Med 2003; 349: 1875–1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

