Viral expression of CCL2 is sufficient to induce demyelination in RAG1-/- mice infected with a neurotropic coronavirus
- PMID: 15890951
- PMCID: PMC1112157
- DOI: 10.1128/JVI.79.11.7113-7120.2005
Viral expression of CCL2 is sufficient to induce demyelination in RAG1-/- mice infected with a neurotropic coronavirus
Abstract
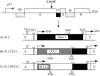
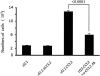
Mouse hepatitis virus strain JHM causes a chronic demyelinating disease in susceptible strains of rodents. Demyelination does not develop in infected RAG1-/- (recombination activation gene-deficient) mice but can be induced by several experimental interventions, including adoptive transfer of virus-specific T cells or antibodies. A common feature of demyelination in these models is extensive infiltration of macrophages/microglia into the white matter. The data obtained thus far do not indicate whether macrophage/microglia infiltration, in the absence of T cells or antibody, is sufficient to mediate demyelination. To determine whether the expression of a single macrophage chemoattractant, in the context of virus infection, could initiate the demyelinating process, we engineered a recombinant coronavirus that expressed the chemokine CCL2/monocyte chemoattractant protein-1. CCL2 has been implicated in macrophage infiltration into the central nervous system and is involved in demyelination in many experimental models of demyelination. Extensive macrophage/microglia infiltration and demyelination has developed in RAG1-/- mice infected with this recombinant virus. Thus, these results suggest that the minimal requirement for demyelination is increased expression of a single macrophage-attracting chemokine in the context of an inflammatory milieu, such as that induced by a viral infection.
Figures

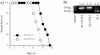

Similar articles
-
CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination.J Immunol. 2000 Aug 15;165(4):2278-86. doi: 10.4049/jimmunol.165.4.2278. J Immunol. 2000. PMID: 10925317
-
Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus.J Virol. 1999 Oct;73(10):8771-80. doi: 10.1128/JVI.73.10.8771-8780.1999. J Virol. 1999. PMID: 10482631 Free PMC article.
-
Contributions of CD8+ T cells and viral spread to demyelinating disease.J Immunol. 2000 Apr 15;164(8):4080-8. doi: 10.4049/jimmunol.164.8.4080. J Immunol. 2000. PMID: 10754301
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
-
Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease.Viral Immunol. 2019 Jan/Feb;32(1):25-37. doi: 10.1089/vim.2018.0073. Epub 2018 Aug 15. Viral Immunol. 2019. PMID: 30109979 Free PMC article. Review.
Cited by
-
The Impact of Innate Components on Viral Pathogenesis in the Neurotropic Coronavirus Encephalomyelitis Mouse Model.Viruses. 2023 Dec 9;15(12):2400. doi: 10.3390/v15122400. Viruses. 2023. PMID: 38140641 Free PMC article. Review.
-
Microglia influence immune responses and restrict neurologic disease in response to central nervous system infection by a neurotropic murine coronavirus.Front Cell Neurosci. 2023 Nov 30;17:1291255. doi: 10.3389/fncel.2023.1291255. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38099152 Free PMC article. Review.
-
Trem2 deficiency impairs recovery and phagocytosis and dysregulates myeloid gene expression during virus-induced demyelination.J Neuroinflammation. 2022 Nov 4;19(1):267. doi: 10.1186/s12974-022-02629-1. J Neuroinflammation. 2022. PMID: 36333761 Free PMC article.
-
Microglia Do Not Restrict SARS-CoV-2 Replication following Infection of the Central Nervous System of K18-Human ACE2 Transgenic Mice.J Virol. 2022 Feb 23;96(4):e0196921. doi: 10.1128/jvi.01969-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935438 Free PMC article.
-
Microglia do not restrict SARS-CoV-2 replication following infection of the central nervous system of K18-hACE2 transgenic mice.bioRxiv [Preprint]. 2021 Nov 17:2021.11.15.468761. doi: 10.1101/2021.11.15.468761. bioRxiv. 2021. Update in: J Virol. 2022 Feb 23;96(4):e0196921. doi: 10.1128/jvi.01969-21. PMID: 34816260 Free PMC article. Updated. Preprint.
References
-
- Banisadr, G., F. Queraud-Lesaux, M. C. Boutterin, D. Pelaprat, B. Zalc, W. Rostene, F. Haour, and S. M. Parsadaniantz. 2002. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J. Neurochem. 81:257-269. - PubMed
-
- Bennett, J. L., A. Elhofy, M. C. Canto, M. Tani, R. M. Ransohoff, and W. J. Karpus. 2003. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45(high)CD11b(+) monocytes and enhanced Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Neurovirol. 9:623-636. - PMC - PubMed
-
- Chen, B. P., W. A. Kuziel, and T. E. Lane. 2001. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 167:4585-4592. - PubMed
-
- Chen, Y., J. M. Hallenbeck, C. Ruetzler, D. Bol, K. Thomas, N. E. Berman, and S. N. Vogel. 2003. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J. Cereb. Blood Flow Metab. 23:748-755. - PubMed
-
- Chua, M. M., K. C. MacNamara, L. San Mateo, H. Shen, and S. R. Weiss. 2004. Effects of an epitope-specific CD8+ T-cell response on murine coronavirus central nervous system disease: protection from virus replication and antigen spread and selection of epitope escape mutants. J. Virol. 78:1150-1159. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

