Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1
- PMID: 15890903
- PMCID: PMC1112142
- DOI: 10.1128/JVI.79.11.6655-6663.2005
Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1
Abstract
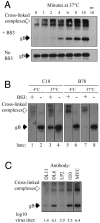
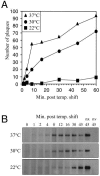
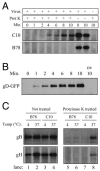
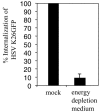
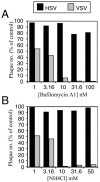
Two herpes simplex virus type 1 (HSV-1) entry pathways have been described: direct fusion between the virion envelope and the plasma membrane, as seen on Vero cells, and low-pH-dependent endocytosis, as seen on CHO nectin-1 and HeLa cells. In this paper, we studied HSV entry into C10 murine melanoma cells and identified a third entry pathway for this virus. During entry into C10 cells, virion envelope glycoproteins rapidly became protected from the membrane-impermeable chemical cross-linker BS3 and from proteinase K. Protection was gD receptor dependent, and the time taken to detect protected protein was proportional to the rate of virus entry. Ultrastructural examination revealed that virions attached to the surface of C10 cells were localized to membrane invaginations, whereas those on the surface of receptor-negative B78 cells were peripherally attached. Virus entry into C10 cells was energy dependent, and intracellular enveloped virions were seen within membrane-bound vesicles consistent with endocytic entry. Entry was not inhibited by bafilomycin A1 or ammonium chloride, showing that passage of the virion through a low-pH environment was not required for infection. Resistance to similar reagents should therefore not be taken as proof of HSV entry by a nonendosomal pathway. These data define a novel gD receptor-dependent acid-independent endocytic entry pathway for HSV.
Figures


Similar articles
-
Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells.Virol J. 2006 Dec 27;3:105. doi: 10.1186/1743-422X-3-105. Virol J. 2006. PMID: 17192179 Free PMC article.
-
Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells.J Virol. 2003 May;77(9):5324-32. doi: 10.1128/jvi.77.9.5324-5332.2003. J Virol. 2003. PMID: 12692234 Free PMC article.
-
Structure-function analysis of herpes simplex virus glycoprotein B with fusion-from-without activity.Virology. 2008 Dec 20;382(2):207-16. doi: 10.1016/j.virol.2008.09.015. Epub 2008 Oct 23. Virology. 2008. PMID: 18950828
-
Herpesvirus Entry into Host Cells Mediated by Endosomal Low pH.Traffic. 2016 Sep;17(9):965-75. doi: 10.1111/tra.12408. Epub 2016 May 24. Traffic. 2016. PMID: 27126894 Free PMC article. Review.
-
Herpes simplex virion entry into and intracellular transport within mammalian cells.Adv Drug Deliv Rev. 2003 Nov 14;55(11):1497-513. doi: 10.1016/j.addr.2003.07.006. Adv Drug Deliv Rev. 2003. PMID: 14597143 Review.
Cited by
-
Evasion of early antiviral responses by herpes simplex viruses.Mediators Inflamm. 2015;2015:593757. doi: 10.1155/2015/593757. Epub 2015 Mar 30. Mediators Inflamm. 2015. PMID: 25918478 Free PMC article. Review.
-
Dissociation of HSV gL from gH by αvβ6- or αvβ8-integrin promotes gH activation and virus entry.Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):E3901-10. doi: 10.1073/pnas.1506846112. Epub 2015 Jul 8. Proc Natl Acad Sci U S A. 2015. PMID: 26157134 Free PMC article.
-
Herpes simplex virus glycoprotein D relocates nectin-1 from intercellular contacts.Virology. 2016 Dec;499:267-277. doi: 10.1016/j.virol.2016.09.019. Epub 2016 Oct 7. Virology. 2016. PMID: 27723487 Free PMC article.
-
Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells.J Virol. 2006 Nov;80(21):10325-34. doi: 10.1128/JVI.00939-06. J Virol. 2006. PMID: 17041213 Free PMC article.
-
Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26.J Virol. 2006 Aug;80(16):8211-24. doi: 10.1128/JVI.02528-05. J Virol. 2006. PMID: 16873277 Free PMC article.
References
-
- Bodaghi, B., M. E. Slobbe-van Drunen, A. Topilko, E. Perret, R. C. Vossen, M. C. van Dam-Mieras, D. Zipeto, J. L. Virelizier, P. LeHoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 40:2598-2607. - PubMed
-
- Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. - PMC - PubMed
-
- Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

