Spatial segregation of gamma-secretase and substrates in distinct membrane domains
- PMID: 15886206
- PMCID: PMC1201532
- DOI: 10.1074/jbc.M503570200
Spatial segregation of gamma-secretase and substrates in distinct membrane domains
Abstract
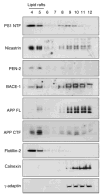
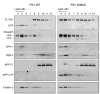
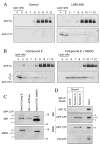
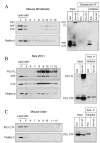
Gamma-secretase facilitates the regulated intramembrane proteolysis of select type I membrane proteins that play diverse physiological roles in multiple cell types and tissue. In this study, we used biochemical approaches to examine the distribution of amyloid precursor protein (APP) and several additional gamma-secretase substrates in membrane microdomains. We report that APP C-terminal fragments (CTFs) and gamma-secretase reside in Lubrol WX detergent-insoluble membranes (DIM) of cultured cells and adult mouse brain. APP CTFs that accumulate in cells lacking gamma-secretase activity preferentially associate with DIM. Cholesterol depletion and magnetic immunoisolation studies indicate recruitment of APP CTFs into cholesterol- and sphingolipid-rich lipid rafts, and co-residence of APP CTFs, PS1, and syntaxin 6 in DIM patches derived from the trans-Golgi network. Photoaffinity cross-linking studies provided evidence for the preponderance of active gamma-secretase in lipid rafts of cultured cells and adult brain. Remarkably, unlike the case of APP, CTFs derived from Notch1, Jagged2, deleted in colorectal cancer (DCC), and N-cadherin remain largely detergent-soluble, indicative of their spatial segregation in non-raft domains. In embryonic brain, the majority of PS1 and nicastrin is present in Lubrol WX-soluble membranes, wherein the CTFs derived from APP, Notch1, DCC, and N-cadherin also reside. We suggest that gamma-secretase residence in non-raft membranes facilitates proteolysis of diverse substrates during embryonic development but that the translocation of gamma-secretase to lipid rafts in adults ensures processing of certain substrates, including APP CTFs, while limiting processing of other potential substrates.
Figures



Similar articles
-
Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes.J Biol Chem. 2004 Oct 22;279(43):44945-54. doi: 10.1074/jbc.M407986200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15322084 Free PMC article.
-
S-palmitoylation of gamma-secretase subunits nicastrin and APH-1.J Biol Chem. 2009 Jan 16;284(3):1373-84. doi: 10.1074/jbc.M806380200. Epub 2008 Nov 20. J Biol Chem. 2009. PMID: 19028695 Free PMC article.
-
A role for presenilin 1 in regulating the delivery of amyloid precursor protein to the cell surface.Neurobiol Dis. 2002 Oct;11(1):64-82. doi: 10.1006/nbdi.2002.0546. Neurobiol Dis. 2002. PMID: 12460547
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
-
The role of proteolysis in Alzheimer's disease.Adv Exp Med Biol. 2000;477:379-90. doi: 10.1007/0-306-46826-3_39. Adv Exp Med Biol. 2000. PMID: 10849764 Review.
Cited by
-
ABCG1 and ABCG4 Suppress γ-Secretase Activity and Amyloid β Production.PLoS One. 2016 May 19;11(5):e0155400. doi: 10.1371/journal.pone.0155400. eCollection 2016. PLoS One. 2016. PMID: 27196068 Free PMC article.
-
Cross-talk of membrane lipids and Alzheimer-related proteins.Mol Neurodegener. 2013 Oct 22;8:34. doi: 10.1186/1750-1326-8-34. Mol Neurodegener. 2013. PMID: 24148205 Free PMC article. Review.
-
HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):247-58. doi: 10.1007/s10911-008-9076-6. Epub 2008 May 13. J Mammary Gland Biol Neoplasia. 2008. PMID: 18473151 Free PMC article. Review.
-
AIBP Limits Angiogenesis Through γ-Secretase-Mediated Upregulation of Notch Signaling.Circ Res. 2017 May 26;120(11):1727-1739. doi: 10.1161/CIRCRESAHA.116.309754. Epub 2017 Mar 21. Circ Res. 2017. PMID: 28325782 Free PMC article.
-
Defective lysosomal proteolysis and axonal transport are early pathogenic events that worsen with age leading to increased APP metabolism and synaptic Abeta in transgenic APP/PS1 hippocampus.Mol Neurodegener. 2012 Nov 22;7:59. doi: 10.1186/1750-1326-7-59. Mol Neurodegener. 2012. PMID: 23173743 Free PMC article.
References
-
- Sisodia SS, St George-Hyslop PH. Nat Rev Neurosci. 2002;3:281–290. - PubMed
-
- Vassar R. J Mol Neurosci. 2004;23:105–114. - PubMed
-
- Iwatsubo T. Curr Opin Neurobiol. 2004;14:379–383. - PubMed
-
- Tanzi RE, Bertram L. Neuron. 2001;32:181–184. - PubMed
-
- Levitan D, Greenwald I. Nature. 1995;377:351–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

