Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY
- PMID: 15870288
- PMCID: PMC1087718
- DOI: 10.1128/MCB.25.10.4189-4199.2005
Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY
Abstract
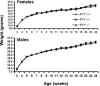
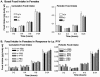
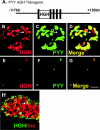
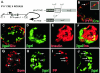
The gastrointestinal hormone peptide YY is a potent inhibitor of food intake and is expressed early during differentiation of intestinal and pancreatic endocrine cells. In order to better understand the role of peptide YY in energy homeostasis and development, we created mice with a targeted deletion of the peptide YY gene. All intestinal and pancreatic endocrine cells developed normally in the absence of peptide YY with the exception of pancreatic polypeptide (PP) cells, indicating that peptide YY expression was not required for terminal differentiation. We used recombination-based cell lineage trace to determine if peptide YY cells were progenitors for gastrointestinal endocrine cells. Peptide YY(+) cells gave rise to all L-type enteroendocrine cells and to islet partial differential and PP cells. In the pancreas, approximately 40% of pancreatic alpha and rare beta cells arose from peptide YY(+) cells, suggesting that most beta cells and surprisingly the majority of alpha cells are not descendants of peptide YY(+)/glucagon-positive/insulin-positive cells that appear during early pancreagenesis. Despite the anorectic effects of exogenous peptide YY(3-36) following intraperitoneal administration, mice lacking peptide YY showed normal growth, food intake, energy expenditure, and responsiveness to peptide YY(3-36). These observations suggest that targeted disruption of the peptide YY gene does not perturb terminal endocrine cell differentiation or the control of food intake and energy homeostasis.
Figures



Similar articles
-
Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor.Development. 1994 Feb;120(2):245-52. doi: 10.1242/dev.120.2.245. Development. 1994. PMID: 8149907
-
Peptide YY expression is an early event in colonic endocrine cell differentiation: evidence from normal and transgenic mice.Development. 1996 Apr;122(4):1157-63. doi: 10.1242/dev.122.4.1157. Development. 1996. PMID: 8620842
-
Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide.Am J Physiol Gastrointest Liver Physiol. 2004 May;286(5):G693-7. doi: 10.1152/ajpgi.00536.2003. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15068960 Review.
-
Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation.Diabetes. 2003 Aug;52(8):2016-24. doi: 10.2337/diabetes.52.8.2016. Diabetes. 2003. PMID: 12882918
-
Ontogeny and the effect of aging on pancreatic polypeptide and peptide YY.Peptides. 2002 Feb;23(2):263-7. doi: 10.1016/s0196-9781(01)00603-9. Peptides. 2002. PMID: 11825641 Review.
Cited by
-
Neuropeptides and their receptors: innovative science providing novel therapeutic targets.Br J Pharmacol. 2006 Jan;147 Suppl 1(Suppl 1):S202-11. doi: 10.1038/sj.bjp.0706461. Br J Pharmacol. 2006. PMID: 16402106 Free PMC article. Review.
-
Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity.Diabetologia. 2006 Jun;49(6):1360-70. doi: 10.1007/s00125-006-0237-0. Epub 2006 Apr 21. Diabetologia. 2006. PMID: 16680491
-
GATA6 is required for proliferation, migration, secretory cell maturation, and gene expression in the mature mouse colon.Mol Cell Biol. 2012 Sep;32(17):3392-402. doi: 10.1128/MCB.00070-12. Epub 2012 Jun 25. Mol Cell Biol. 2012. PMID: 22733991 Free PMC article.
-
Regulation of hindbrain Pyy expression by acute food deprivation, prolonged caloric restriction, and weight loss surgery in mice.Am J Physiol Endocrinol Metab. 2012 Sep 1;303(5):E659-68. doi: 10.1152/ajpendo.00033.2012. Epub 2012 Jul 3. Am J Physiol Endocrinol Metab. 2012. PMID: 22761162 Free PMC article.
-
Transcriptomic analysis of the lesser spotted catshark (Scyliorhinus canicula) pancreas, liver and brain reveals molecular level conservation of vertebrate pancreas function.BMC Genomics. 2014 Dec 6;15:1074. doi: 10.1186/1471-2164-15-1074. BMC Genomics. 2014. PMID: 25480530 Free PMC article.
References
-
- Adams, S. H., W. B. Won, S. E. Schonhoff, A. B. Leiter, and J. R. Paterniti, Jr. 2004. Effects of peptide YY[3-36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology 145:4967-4975. - PubMed
-
- Asakawa, A., A. Inui, N. Ueno, M. Fujimiya, M. A. Fujino, and M. Kasuga. 1999. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides 20:1445-1448. - PubMed
-
- Batterham, R. L., M. A. Cowley, C. J. Small, H. Herzog, M. A. Cohen, C. L. Dakin, A. M. Wren, A. E. Brynes, M. J. Low, M. A. Ghatei, R. D. Cone, and S. R. Bloom. 2002. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650-654. - PubMed
-
- Batterham, R. L., C. W. Le Roux, M. A. Cohen, A. J. Park, S. M. Ellis, M. Patterson, G. S. Frost, M. A. Ghatei, and S. R. Bloom. 2003. Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 88:3989-3992. - PubMed
-
- Brouns, I., L. Van Nassauw, J. Van Genechten, M. Majewski, D. W. Scheuermann, J. P. Timmermans, and D. Adriaensen. 2002. Triple immunofluorescence staining with antibodies raised in the same species to study the complex innervation pattern of intrapulmonary chemoreceptors. J. Histochem. Cytochem. 50:575-582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- U19 DK042502/DK/NIDDK NIH HHS/United States
- P30 DK034928/DK/NIDDK NIH HHS/United States
- DK67166/DK/NIDDK NIH HHS/United States
- DK52870/DK/NIDDK NIH HHS/United States
- T32-CA65441/CA/NCI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30-DK34928/DK/NIDDK NIH HHS/United States
- DK43673/DK/NIDDK NIH HHS/United States
- T32 CA065441/CA/NCI NIH HHS/United States
- DK42502/DK/NIDDK NIH HHS/United States
- R01 DK067166/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- P01 DK042502/DK/NIDDK NIH HHS/United States
- R01 DK043673/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
