Phosphorylation by Cak1 regulates the C-terminal domain kinase Ctk1 in Saccharomyces cerevisiae
- PMID: 15870265
- PMCID: PMC1087728
- DOI: 10.1128/MCB.25.10.3906-3913.2005
Phosphorylation by Cak1 regulates the C-terminal domain kinase Ctk1 in Saccharomyces cerevisiae
Abstract
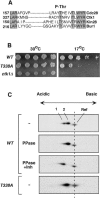
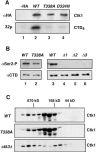
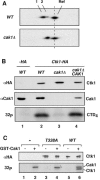
Ctk1 is a Saccharomyces cerevisiae cyclin-dependent protein kinase (CDK) that assembles with Ctk2 and Ctk3 to form an active protein kinase complex, CTDK-I. CTDK-I phosphorylates Ser2 within the RNA polymerase II C-terminal domain, an activity that is required for efficient transcriptional elongation and 3' RNA processing. Ctk1 contains a conserved T loop, which undergoes activating phosphorylation in other CDKs. We show that Ctk1 is phosphorylated on Thr-338 within the T loop. Mutation of this residue abolished Ctk1 kinase activity in vitro and resulted in a cold-sensitive phenotype. As with other yeast CDKs undergoing T-loop phosphorylation, Ctk1 phosphorylation on Thr-338 was dependent on the Cak1 protein kinase. Ctk1 isolated from cak1Delta cells was unphosphorylated and exhibited low protein kinase activity. Moreover, Cak1 directly phosphorylated Ctk1 in vitro. Unlike wild-type cells, cells expressing Ctk1(T338A) delayed growth at early stationary phase, did not show the increase in Ser2 phosphorylation that normally accompanies the transition from rapid growth to stationary phase, and had compromised transcriptional activation of two stationary-phase genes, CTT1 and SPI1. Therefore, Ctk1 phosphorylation on Thr-338 is carried out by Cak1 and is required for normal gene transcription during the transition into stationary phase.
Figures


Similar articles
-
A feed forward circuit comprising Spt6, Ctk1 and PAF regulates Pol II CTD phosphorylation and transcription elongation.Nucleic Acids Res. 2014 Jan;42(2):870-81. doi: 10.1093/nar/gkt1003. Epub 2013 Oct 25. Nucleic Acids Res. 2014. PMID: 24163256 Free PMC article.
-
Kinase Cak1 functionally interacts with the PAF1 complex and phosphatase Ssu72 via kinases Ctk1 and Bur1.Mol Genet Genomics. 2006 Feb;275(2):136-47. doi: 10.1007/s00438-005-0071-y. Epub 2005 Dec 1. Mol Genet Genomics. 2006. PMID: 16362371
-
CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1.Genes Dev. 2010 Oct 15;24(20):2303-16. doi: 10.1101/gad.1968210. Genes Dev. 2010. PMID: 20952539 Free PMC article.
-
Bur1/Bur2 and the Ctk complex in yeast: the split personality of mammalian P-TEFb.Cell Cycle. 2006 May;5(10):1066-8. doi: 10.4161/cc.5.10.2769. Epub 2006 May 15. Cell Cycle. 2006. PMID: 16721054 Review.
-
RNA polymerase II transcription elongation and Pol II CTD Ser2 phosphorylation: A tail of two kinases.Nucleus. 2014 May-Jun;5(3):224-36. doi: 10.4161/nucl.29347. Epub 2014 May 30. Nucleus. 2014. PMID: 24879308 Free PMC article. Review.
Cited by
-
Mechanisms regulating the protein kinases of Saccharomyces cerevisiae.Eukaryot Cell. 2007 Apr;6(4):571-83. doi: 10.1128/EC.00026-07. Epub 2007 Mar 2. Eukaryot Cell. 2007. PMID: 17337635 Free PMC article. Review. No abstract available.
-
Cdk7: a kinase at the core of transcription and in the crosshairs of cancer drug discovery.Transcription. 2019 Apr;10(2):47-56. doi: 10.1080/21541264.2018.1553483. Epub 2018 Dec 6. Transcription. 2019. PMID: 30488763 Free PMC article. Review.
-
Pseudosubstrate inhibition of the anaphase-promoting complex by Acm1: regulation by proteolysis and Cdc28 phosphorylation.Mol Cell Biol. 2008 Aug;28(15):4653-64. doi: 10.1128/MCB.00055-08. Epub 2008 Jun 2. Mol Cell Biol. 2008. PMID: 18519589 Free PMC article.
-
Ascospore formation in the yeast Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2005 Dec;69(4):565-84. doi: 10.1128/MMBR.69.4.565-584.2005. Microbiol Mol Biol Rev. 2005. PMID: 16339736 Free PMC article. Review.
-
Identification of yeast IQGAP (Iqg1p) as an anaphase-promoting-complex substrate and its role in actomyosin-ring-independent cytokinesis.Mol Biol Cell. 2007 Dec;18(12):5139-53. doi: 10.1091/mbc.e07-05-0509. Epub 2007 Oct 17. Mol Biol Cell. 2007. PMID: 17942599 Free PMC article.
References
-
- Ahn, S. H., M. Kim, and S. Buratowski. 2004. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13:67-76. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Borggrefe, T., R. Davis, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 2002. A complex of the srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 277:44202-44207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
