Persistence of Streptococcus pyogenes in stationary-phase cultures
- PMID: 15866916
- PMCID: PMC1111994
- DOI: 10.1128/JB.187.10.3319-3328.2005
Persistence of Streptococcus pyogenes in stationary-phase cultures
Abstract
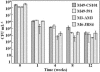
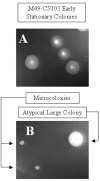
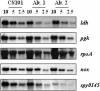
In addition to causing fulminant disease, Streptococcus pyogenes may be asymptomatically carried between recurrent episodes of pharyngitis. To better understand streptococcal carriage, we characterized in vitro long-term stationary-phase survival (>4 weeks) of S. pyogenes. When grown in sugar-limited Todd-Hewitt broth, S. pyogenes cells remained culturable for more than 1 year. Both Todd-Hewitt supplemented with excess glucose and chemically defined medium allowed survival for less than 1 week. After 4 weeks of survival in sugar-limited Todd-Hewitt broth, at least 10(3) CFU per ml remained. When stained with fluorescent live-dead viability stain, there were a number of cells with intact membranes that were nonculturable. Under conditions that did not support persistence, these cells disappeared 2 weeks after loss of culturability. In persistent cultures, these may be cells that are dying during cell turnover. After more than 4 weeks in stationary phase, the culturable cells formed two alternative colony phenotypes: atypical large colonies and microcolonies. Protein expression in two independently isolated microcolony strains, from 14-week cultures, was examined by use of two-dimensional electrophoresis. The proteomes of these two strains exhibited extensive changes compared to the parental strain. While some of these changes were common to the two strains, many of the changes were unique to a single strain. Some of the common changes were in metabolic pathways, suggesting a possible alternate metabolism for the persisters. Overall, these data suggest that under certain in vitro conditions, S. pyogenes cells can persist for greater than 1 year as a dynamic population.
Figures

Similar articles
-
H(2)O(2)-nonproducing Streptococcus pyogenes strains: survival in stationary phase and virulence in chronic granulomatous disease.Microbiology (Reading). 2001 Sep;147(Pt 9):2469-2477. doi: 10.1099/00221287-147-9-2469. Microbiology (Reading). 2001. PMID: 11535787
-
Survival of Streptococcus pyogenes under stress and starvation.FEMS Microbiol Lett. 1999 Jul 15;176(2):421-8. doi: 10.1111/j.1574-6968.1999.tb13692.x. FEMS Microbiol Lett. 1999. PMID: 10427725
-
Generation of metabolically diverse strains of Streptococcus pyogenes during survival in stationary phase.J Bacteriol. 2009 Oct;191(20):6242-52. doi: 10.1128/JB.00440-09. Epub 2009 Aug 7. J Bacteriol. 2009. PMID: 19666718 Free PMC article.
-
Pediatric pharyngitis.Pediatr Case Rev. 2003 Oct;3(4):203-10. doi: 10.1097/01.PCA.0000085283.22184.DF. Pediatr Case Rev. 2003. PMID: 14520082 Review. No abstract available.
-
The Streptococcus pyogenes Proteome.2022 Jul 22. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 11. 2022 Jul 22. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 11. PMID: 36479765 Free Books & Documents. Review. No abstract available.
Cited by
-
Kinetic and structural characterization for cofactor preference of succinic semialdehyde dehydrogenase from Streptococcus pyogenes.Mol Cells. 2014 Oct 31;37(10):719-26. doi: 10.14348/molcells.2014.0162. Epub 2014 Sep 26. Mol Cells. 2014. PMID: 25256219 Free PMC article.
-
DNA-based culture-independent analysis detects the presence of group a streptococcus in throat samples from healthy adults in Japan.BMC Microbiol. 2016 Oct 11;16(1):237. doi: 10.1186/s12866-016-0858-5. BMC Microbiol. 2016. PMID: 27724855 Free PMC article.
-
Laboratory growth and maintenance of Streptococcus pyogenes (the Group A Streptococcus, GAS).Curr Protoc Microbiol. 2013 Oct 2;30:9D.2.1-9D.2.13. doi: 10.1002/9780471729259.mc09d02s30. Curr Protoc Microbiol. 2013. PMID: 24510893 Free PMC article.
-
Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12147-52. doi: 10.1073/pnas.1203735109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778419 Free PMC article.
-
Corynebacterium jeikeium Dormant Cell Formation and Photodynamic Inactivation.Front Microbiol. 2020 Dec 18;11:605899. doi: 10.3389/fmicb.2020.605899. eCollection 2020. Front Microbiol. 2020. PMID: 33391228 Free PMC article.
References
-
- Audia, J. P., C. C. Webb, and J. W. Foster. 2001. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int. J. Med. Microbiol. 291:97-106. - PubMed
-
- Brandt, C. M., F. Allerberger, B. Spellerberg, R. Holland, R. Lutticken, and G. Haase. 2001. Characterization of consecutive Streptococcus pyogenes isolates from patients with pharyngitis and bacteriological treatment failure: special reference to prtF1 and sic / drs. J. Infect. Dis. 183:670-674. - PubMed
-
- Buttner, K., J. Bernhardt, C. Scharf, R. Schmid, U. Mader, C. Eymann, H. Antelmann, A. Volker, U. Volker, and M. Hecker. 2001. A comprehensive two-dimensional map of cytosolic proteins of Bacillus subtilis. Electrophoresis 22:2908-2935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

