COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells
- PMID: 15849318
- PMCID: PMC1084325
- DOI: 10.1093/nar/gki529
COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells
Retraction in
-
Retraction of 'COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells'.Nucleic Acids Res. 2023 May 9;51(10):5300. doi: 10.1093/nar/gkad358. Online ahead of print. Nucleic Acids Res. 2023. PMID: 37156599 Free PMC article. No abstract available.
Expression of concern in
-
Expression of concern on 'COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells'.Nucleic Acids Res. 2022 Nov 28;50(21):12600. doi: 10.1093/nar/gkac1167. Nucleic Acids Res. 2022. Retraction in: Nucleic Acids Res. 2023 May 09:gkad358. doi: 10.1093/nar/gkad358 PMID: 36440763 Free PMC article. Retracted. No abstract available.
Abstract
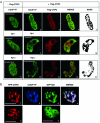
Human immunodeficiency virus type 1 (HIV-1) gene transcription is characterized by two temporally distinct phases. While the initial phase relies solely on cellular transcription factors, the subsequent phase is activated by the viral Tat transactivator. We have previously reported that the subsequent phase of viral gene transcription can be repressed by the chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2 (CTIP2) in human microglial cells [O. Rohr, D. Lecestre, S. Chasserot-Golaz, C. Marban, D. Avram, D. Aunis, M. Leid and E. Schaeffer (2003), J. Virol., 77, 5415-5427]. Here, we demonstrate that CTIP proteins also repress the initial phase of HIV-1 gene transcription, mainly supported by the cellular transcription factors Sp1 and COUP-TF in microglial cells. We report that CTIP2 represses Sp1- and COUP-TF-mediated activation of HIV-1 gene transcription and viral replication as a result of physical interactions with COUP-TF and Sp1 in microglial nuclei. Using laser confocal microscopy CTIP2 was found to colocalize with Sp1, COUP-TF and the heterochromatin-associated protein Hp1alpha, which is mainly detected in transcriptionally repressed heterochromatic region. Moreover, we describe that CTIP2 can be recruited to the HIV-1 promoter via its association with Sp1 bound to the GC-box sequences of the long terminal repeat (LTR). Since our findings demonstrate that CTIP2 interacts with the HIV-1 proximal promoter, it is likely that CTIP2 promotes HIV-1 gene silencing by forcing transcriptionally repressed heterochromatic environment to the viral LTR region.
Figures


Similar articles
-
Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression.J Biol Chem. 2003 Oct 31;278(44):43041-50. doi: 10.1074/jbc.M307477200. Epub 2003 Aug 19. J Biol Chem. 2003. PMID: 12930829 Free PMC article.
-
Recruitment of Tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells.J Virol. 2003 May;77(9):5415-27. doi: 10.1128/jvi.77.9.5415-5427.2003. J Virol. 2003. PMID: 12692243 Free PMC article.
-
COUP-TF and Sp1 interact and cooperate in the transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat in human microglial cells.J Biol Chem. 1997 Dec 5;272(49):31149-55. doi: 10.1074/jbc.272.49.31149. J Biol Chem. 1997. PMID: 9388268
-
Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions.Keio J Med. 2003 Sep;52(3):174-81. doi: 10.2302/kjm.52.174. Keio J Med. 2003. PMID: 14529150 Review.
-
Regulation of differential COUP-TF-coregulator interactions in adrenal cortical steroidogenesis.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):449-56. doi: 10.1016/s0960-0760(03)00217-6. J Steroid Biochem Mol Biol. 2003. PMID: 12943735 Review.
Cited by
-
Zinc Finger Protein BCL11A Contributes to the Abortive Infection of Hirame novirhabdovirus (HIRRV) in B Lymphocytes of Flounder (Paralichthys olivaceus).J Virol. 2022 Dec 21;96(24):e0147022. doi: 10.1128/jvi.01470-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448803 Free PMC article.
-
Epigenetic regulation of HIV-1 latency by cytosine methylation.PLoS Pathog. 2009 Jun;5(6):e1000495. doi: 10.1371/journal.ppat.1000495. Epub 2009 Jun 26. PLoS Pathog. 2009. PMID: 19557157 Free PMC article.
-
A de novo substitution in BCL11B leads to loss of interaction with transcriptional complexes and craniosynostosis.Hum Mol Genet. 2019 Aug 1;28(15):2501-2513. doi: 10.1093/hmg/ddz072. Hum Mol Genet. 2019. PMID: 31067316 Free PMC article.
-
HIV latency: experimental systems and molecular models.FEMS Microbiol Rev. 2012 May;36(3):706-16. doi: 10.1111/j.1574-6976.2012.00335.x. FEMS Microbiol Rev. 2012. PMID: 22372374 Free PMC article. Review.
-
Regulation of Expression and Latency in BLV and HTLV.Viruses. 2020 Sep 25;12(10):1079. doi: 10.3390/v12101079. Viruses. 2020. PMID: 32992917 Free PMC article. Review.
References
-
- Fauci A.S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. - PubMed
-
- Portegies P., Brew B.J. Update on HIV-related neurological illness. AIDS. 1991;5(Suppl. 2):S211–S217. - PubMed
-
- Pialoux G., Fournier S., Moulignier A., Poveda J.D., Clavel F., Dupont B. Central nervous system as a sanctuary for HIV-1 infection despite treatment with zidovudine, lamivudine and indinavir. AIDS. 1997;11:1302–1303. - PubMed
-
- Kolson D.L., Lavi E., Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 1998;50:1–47. - PubMed
-
- Peudenier S., Hery C., Montagnier L., Tardieu M. Human microglial cells: characterization in cerebral tissue and in primary culture, and study of their susceptibility to HIV-1 infection. Ann. Neurol. 1991;29:152–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

