Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter
- PMID: 15827205
- PMCID: PMC1082719
- DOI: 10.1128/JVI.79.9.5880-5885.2005
Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter
Abstract
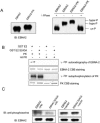
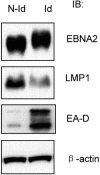
The Epstein-Barr virus (EBV) BGLF4 gene encodes a serine/threonine protein kinase (PK) that is expressed in the cytolytic cycle. EBV nuclear antigen 2 (EBNA2) is a key latency gene essential for immortalization of B lymphocytes and transactivation of viral and cellular promoters. Here we report that EBV PK phosphorylates EBNA2 at Ser-243 and that these two proteins physically associate. PK suppresses EBNA2's ability to transactivate the LMP1 promoter, and Ser-243 of EBNA2 is involved in this suppression. Moreover, EBNA2 is hyperphosphorylated during EBV reactivation in latently infected B cells, which is associated with decreased LMP1 protein levels. This is the first report about the effect of EBV PK on the function of one of its target proteins and regulation of EBNA2 phosphorylation during the EBV lytic cycle.
Figures

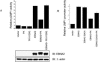
Similar articles
-
cdc2/cyclin B1-dependent phosphorylation of EBNA2 at Ser243 regulates its function in mitosis.J Virol. 2006 Feb;80(4):2045-50. doi: 10.1128/JVI.80.4.2045-2050.2006. J Virol. 2006. PMID: 16439560 Free PMC article.
-
The NP9 protein encoded by the human endogenous retrovirus HERV-K(HML-2) negatively regulates gene activation of the Epstein-Barr virus nuclear antigen 2 (EBNA2).Int J Cancer. 2011 Sep 1;129(5):1105-15. doi: 10.1002/ijc.25760. Epub 2011 Jan 12. Int J Cancer. 2011. PMID: 21710493
-
Epstein-Barr virus-derived EBNA2 regulates STAT3 activation.Biochem Biophys Res Commun. 2009 Jan 16;378(3):439-43. doi: 10.1016/j.bbrc.2008.11.053. Epub 2008 Nov 24. Biochem Biophys Res Commun. 2009. PMID: 19032945
-
Both Epstein-Barr viral nuclear antigen 2 (EBNA2) and activated Notch1 transactivate genes by interacting with the cellular protein RBP-J kappa.Immunobiology. 1997 Dec;198(1-3):299-306. doi: 10.1016/s0171-2985(97)80050-2. Immunobiology. 1997. PMID: 9442401 Review.
-
EBNA2 and Its Coactivator EBNA-LP.Curr Top Microbiol Immunol. 2015;391:35-59. doi: 10.1007/978-3-319-22834-1_2. Curr Top Microbiol Immunol. 2015. PMID: 26428371 Review.
Cited by
-
EBNA2 interferes with the germinal center phenotype by downregulating BCL6 and TCL1 in non-Hodgkin's lymphoma cells.J Virol. 2007 Mar;81(5):2274-82. doi: 10.1128/JVI.01822-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151114 Free PMC article.
-
The regulatory role of protein phosphorylation in human gammaherpesvirus associated cancers.Virol Sin. 2017 Oct;32(5):357-368. doi: 10.1007/s12250-017-4081-9. Epub 2017 Oct 30. Virol Sin. 2017. PMID: 29116588 Free PMC article. Review.
-
The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production.J Virol. 2009 Nov;83(21):10877-91. doi: 10.1128/JVI.00525-09. Epub 2009 Aug 26. J Virol. 2009. PMID: 19710145 Free PMC article.
-
PLK1-dependent phosphorylation restrains EBNA2 activity and lymphomagenesis in EBV-infected mice.EMBO Rep. 2021 Dec 6;22(12):e53007. doi: 10.15252/embr.202153007. Epub 2021 Oct 4. EMBO Rep. 2021. PMID: 34605140 Free PMC article.
-
Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II.J Virol. 2007 May;81(10):5166-80. doi: 10.1128/JVI.00120-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360754 Free PMC article.
References
-
- Chee, M., G. Lawrence, and B. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

