The nitric oxide donor sodium nitroprusside stimulates the Na+-K+ pump in isolated rabbit cardiac myocytes
- PMID: 15817632
- PMCID: PMC1464570
- DOI: 10.1113/jphysiol.2005.086447
The nitric oxide donor sodium nitroprusside stimulates the Na+-K+ pump in isolated rabbit cardiac myocytes
Abstract
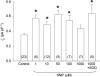
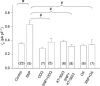
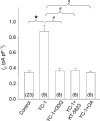
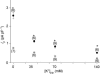
Nitric oxide (NO) affects the membrane Na(+)-K(+) pump in a tissue-dependent manner. Stimulation of intrinsic pump activity, stimulation secondary to NO-induced Na(+) influx into cells or inhibition has been reported. We used the whole-cell patch clamp technique to measure electrogenic Na(+)-K(+) pump current (I(p)) in rabbit ventricular myocytes. Myocytes were voltage clamped with wide-tipped patch pipettes to achieve optimal perfusion of the intracellular compartment, and I(p) was identified as the shift in holding current induced by 100 microm ouabain. The NO donor sodium nitroprusside (SNP) in concentrations of 1, 10, 50 or 100 microm induced a significant increase in I(p) when the intracellular compartment was perfused with pipette solutions containing 10 mm Na(+), a concentration near physiological levels. SNP had no effect when the pump was near-maximally activated by 80 mm Na(+) in pipette solutions. Stimulation persisted in the absence of extracellular Na(+), indicating its independence of transmembrane Na(+) influx. The SNP-induced pump stimulation was abolished by inhibition of soluble guanylyl cyclase (sGC) with 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one, by inhibition of protein kinase G (PKG) with KT-5823 or by inhibition of protein phosphatase with okadaic acid. Inclusion of the non-hydrolysable cGMP analogue 8pCPT-cGMP, activated recombinant PKG or the sGC-activator YC-1 in patch pipette filling solutions reproduced the SNP-induced pump stimulation. Pump stimulation induced by YC-1 was dependent on the Na(+) concentration but not the K(+) concentration in pipette filling solutions, suggesting an altered sensitivity of the Na(+)-K(+) pump to intracellular Na(+).
Figures




Similar articles
-
Natriuretic peptides stimulate the cardiac sodium pump via NPR-C-coupled NOS activation.Am J Physiol Cell Physiol. 2008 Apr;294(4):C1067-73. doi: 10.1152/ajpcell.00243.2007. Epub 2008 Feb 13. Am J Physiol Cell Physiol. 2008. PMID: 18272821
-
Opposing effects of coupled and uncoupled NOS activity on the Na+-K+ pump in cardiac myocytes.Am J Physiol Cell Physiol. 2008 Feb;294(2):C572-8. doi: 10.1152/ajpcell.00242.2007. Epub 2007 Dec 5. Am J Physiol Cell Physiol. 2008. PMID: 18057120
-
Stimulation of the cardiac myocyte Na+-K+ pump due to reversal of its constitutive oxidative inhibition.Am J Physiol Cell Physiol. 2015 Aug 15;309(4):C239-50. doi: 10.1152/ajpcell.00392.2014. Epub 2015 Jun 17. Am J Physiol Cell Physiol. 2015. PMID: 26084308 Free PMC article.
-
Redox-dependent regulation of the Na⁺-K⁺ pump: new twists to an old target for treatment of heart failure.J Mol Cell Cardiol. 2013 Aug;61:94-101. doi: 10.1016/j.yjmcc.2013.05.013. Epub 2013 May 30. J Mol Cell Cardiol. 2013. PMID: 23727392 Review.
-
Greasing the wheels or a spanner in the works? Regulation of the cardiac sodium pump by palmitoylation.Crit Rev Biochem Mol Biol. 2018 Apr;53(2):175-191. doi: 10.1080/10409238.2018.1432560. Epub 2018 Feb 9. Crit Rev Biochem Mol Biol. 2018. PMID: 29424237 Review.
Cited by
-
Nitric oxide mediates the vagal protective effect on ventricular fibrillation via effects on action potential duration restitution in the rabbit heart.J Physiol. 2007 Sep 1;583(Pt 2):695-704. doi: 10.1113/jphysiol.2007.138461. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627986 Free PMC article.
-
Activation of cAMP-dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity.J Biol Chem. 2010 Apr 30;285(18):13712-20. doi: 10.1074/jbc.M109.090225. Epub 2010 Mar 1. J Biol Chem. 2010. PMID: 20194511 Free PMC article.
-
Nicorandil stimulates a Na⁺/Ca²⁺ exchanger by activating guanylate cyclase in guinea pig cardiac myocytes.Pflugers Arch. 2016 Apr;468(4):693-703. doi: 10.1007/s00424-015-1763-8. Epub 2015 Dec 3. Pflugers Arch. 2016. PMID: 26631169
-
Susceptibility of β1 Na+-K+ pump subunit to glutathionylation and oxidative inhibition depends on conformational state of pump.J Biol Chem. 2012 Apr 6;287(15):12353-64. doi: 10.1074/jbc.M112.340893. Epub 2012 Feb 21. J Biol Chem. 2012. PMID: 22354969 Free PMC article.
-
Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease.Int J Mol Sci. 2023 Apr 26;24(9):7855. doi: 10.3390/ijms24097855. Int J Mol Sci. 2023. PMID: 37175562 Free PMC article. Review.
References
-
- Apell HJ, Karlish SJ. Functional properties of Na,K-ATPase, and their structural implications, as detected with biophysical techniques. J Membr Biol. 2001;180:1–9. - PubMed
-
- Buhagiar KA, Hansen PS, Gray DF, Mihailidou AS, Rasmussen HH. Angiotensin regulates the selectivity of the Na+-K+ pump for intracellular Na+ Am J Physiol. 1999;277:C461–C468. - PubMed
-
- Buhagiar KA, Hansen PS, Kong BY, Clarke RJ, Fernandes C, Rasmussen HH. Dietary cholesterol alters Na+/K+ selectivity at intracellular Na+/K+ pump sites in cardiac myocytes. Am J Physiol. 2004;286:C398–C405. - PubMed
-
- Cornelius F, Mahmmoud YA, Christensen HR. Modulation of Na,K-ATPase by associated small transmembrane regulatory proteins and by lipids. J Bioenerg Biomembr. 2001;33:415–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

