Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration
- PMID: 15814784
- PMCID: PMC6725385
- DOI: 10.1523/JNEUROSCI.0081-05.2005
Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration
Abstract
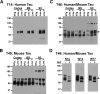
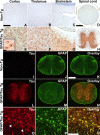
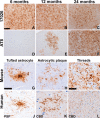
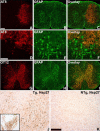
Filamentous tau inclusions in neurons and glia are neuropathological hallmarks of sporadic and familial tauopathies. Because tau gene mutations are pathogenic for the autosomal dominant tauopathy "frontotemporal dementia and parkinsonism linked to chromosome 17," tau abnormalities are implicated directly in the onset and/or progression of disease. Although filamentous tau aggregates are acknowledged to play roles in degenerative mechanisms resulting in neuron loss, the contributions of glial tau pathology to neurodegeneration remain essentially unexplored. To begin to elucidate the role of glial pathology in tauopathies, we generated a transgenic (Tg) mouse model of astrocytic tau pathology by expressing the human tau protein driven by the glial fibrillary acidic protein (GFAP) promoter. Whereas endogenous tau was not detected in astrocytes of control mice, in GFAP/tau Tg mice there was robust astrocytic tau expression that was associated with a redistribution of the GFAP network. Subsequently, there was an age-dependent accumulation of tau pathology in astrocytes that was Gallyas and variably thioflavine S positive as observed in many tauopathies. The tau pathology in these Tg mice was abnormally phosphorylated, ubiquitinated, and filamentous, and the emergence of this pathology coincided with accumulation of insoluble tau protein. Furthermore, in regions with robust astrocytic tau pathology, there was mild blood- brain barrier disruption, induction of low-molecular-weight heat shock proteins, and focal neuron degeneration. Thus, these Tg mice recapitulate key features of astrocytic pathology observed in human tauopathies and demonstrate functional consequences of this pathology including neuron degeneration in the absence of neuronal tau inclusions.
Figures




Similar articles
-
Impaired glutamate transport in a mouse model of tau pathology in astrocytes.J Neurosci. 2006 Jan 11;26(2):644-54. doi: 10.1523/JNEUROSCI.3861-05.2006. J Neurosci. 2006. PMID: 16407562 Free PMC article.
-
Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration.Neuron. 2002 Aug 1;35(3):433-46. doi: 10.1016/s0896-6273(02)00789-4. Neuron. 2002. PMID: 12165467
-
Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau-transgenic mouse.Brain Res. 2011 Sep 2;1410:12-23. doi: 10.1016/j.brainres.2011.06.064. Epub 2011 Jul 7. Brain Res. 2011. PMID: 21803337
-
[Neuropathology of tauopathy].Brain Nerve. 2013 Dec;65(12):1445-58. Brain Nerve. 2013. PMID: 24323931 Review. Japanese.
-
Transgenic mouse models of tauopathies: prospects for animal models of Pick's disease.Neurology. 2001 Jun;56(11 Suppl 4):S26-30. doi: 10.1212/wnl.56.suppl_4.s26. Neurology. 2001. PMID: 11402147 Review.
Cited by
-
Toward an animal model of Progressive Supranuclear Palsy.Front Neurosci. 2024 Oct 3;18:1433465. doi: 10.3389/fnins.2024.1433465. eCollection 2024. Front Neurosci. 2024. PMID: 39420986 Free PMC article. Review.
-
Animal models for Alzheimer's disease and frontotemporal dementia: a perspective.ASN Neuro. 2009 Nov 9;1(4):e00019. doi: 10.1042/AN20090042. ASN Neuro. 2009. PMID: 19839939 Free PMC article.
-
Developmental Pathogenicity of 4-Repeat Human Tau Is Lost with the P301L Mutation in Genetically Matched Tau-Transgenic Mice.J Neurosci. 2020 Jan 2;40(1):220-236. doi: 10.1523/JNEUROSCI.1256-19.2019. Epub 2019 Nov 4. J Neurosci. 2020. PMID: 31685653 Free PMC article.
-
A Closer Look into the Role of Protein Tau in the Identification of Promising Therapeutic Targets for Alzheimer's Disease.Brain Sci. 2018 Aug 26;8(9):162. doi: 10.3390/brainsci8090162. Brain Sci. 2018. PMID: 30149687 Free PMC article. Review.
-
Rett syndrome: what do we know for sure?Nat Neurosci. 2009 Mar;12(3):239-40. doi: 10.1038/nn0309-239. Nat Neurosci. 2009. PMID: 19238181 No abstract available.
References
-
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11: 153-163. - PubMed
-
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10: 1089-1099. - PubMed
-
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27: 117-120. - PubMed
-
- Claudio L (1996) Ultrastructural features of the blood-brain barrier in biopsy tissue from Alzheimer's disease patients. Acta Neuropathol (Berl) 91: 6-14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
