Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction
- PMID: 15809374
- PMCID: PMC3733536
- DOI: 10.1161/01.CIR.0000160936.91849.9F
Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction
Abstract
Background: We used a molecular probe activated by protease cleavage to image expression of matrix metalloproteinases (MMPs) in the heart after myocardial infarction.
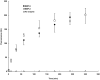
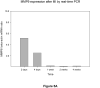
Methods and results: We synthesized and characterized a near-infrared fluorescent (NIRF) probe that is activated by proteolytic cleavage by MMP2 and MMP9. The NIRF probe was injected into mice at various time points up to 4 weeks after myocardial infarction induced by ligation of the left anterior descending coronary artery. NIRF imaging of MMP activity increased in the infarct region, with maximal expression at 1 to 2 weeks, persisting to 4 weeks. Zymography and real-time polymerase chain reaction analysis showed that MMP9 expression is increased at 2 to 4 days, and MMP2 expression is increased at 1 to 2 weeks. Dual-label confocal microscopy showed colocalization of NIRF imaging with neutrophils on day 2, and flow cytometric analysis confirmed that NIRF signal is associated with leukocytes in the infarct zone.
Conclusions: This study demonstrates that the activity of MMPs in the myocardium may be imaged by use of specific activity-dependent molecular probes.
Figures







Comment in
-
Molecular beacons illuminate subcellular events.Circulation. 2005 Apr 12;111(14):1730-2. doi: 10.1161/01.CIR.0000162487.80640.AB. Circulation. 2005. PMID: 15824208 No abstract available.
Similar articles
-
In vivo near-infrared fluorescence imaging of matrix metalloproteinase activity after cerebral ischemia.J Cereb Blood Flow Metab. 2009 Jul;29(7):1284-92. doi: 10.1038/jcbfm.2009.51. Epub 2009 May 6. J Cereb Blood Flow Metab. 2009. PMID: 19417756
-
Imaging of MMP activity in postischemic cardiac remodeling using radiolabeled MMP-2/9 activatable peptide probes.Mol Pharm. 2014 May 5;11(5):1415-23. doi: 10.1021/mp400569k. Epub 2014 Apr 9. Mol Pharm. 2014. PMID: 24641497
-
TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation.Circ Res. 2010 Mar 5;106(4):796-808. doi: 10.1161/CIRCRESAHA.109.209189. Epub 2010 Jan 7. Circ Res. 2010. PMID: 20056917
-
Matrix Metalloproteinases in Myocardial Infarction and Heart Failure.Prog Mol Biol Transl Sci. 2017;147:75-100. doi: 10.1016/bs.pmbts.2017.02.001. Epub 2017 Mar 18. Prog Mol Biol Transl Sci. 2017. PMID: 28413032 Free PMC article. Review.
-
Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window.Cardiovasc Res. 2006 Feb 15;69(3):604-13. doi: 10.1016/j.cardiores.2005.10.002. Epub 2005 Dec 19. Cardiovasc Res. 2006. PMID: 16360129 Review.
Cited by
-
In vivo optical molecular imaging of matrix metalloproteinase activity in abdominal aortic aneurysms correlates with treatment effects on growth rate.Atherosclerosis. 2010 Sep;212(1):181-7. doi: 10.1016/j.atherosclerosis.2010.05.012. Epub 2010 May 24. Atherosclerosis. 2010. PMID: 20542274 Free PMC article.
-
Molecular imaging of interstitial alterations after myocardial infarction.J Cardiovasc Transl Res. 2008 Sep;1(3):221-4. doi: 10.1007/s12265-008-9035-z. Epub 2008 Jun 28. J Cardiovasc Transl Res. 2008. PMID: 20559923
-
Substrate-based near-infrared imaging sensors enable fluorescence lifetime contrast via built-in dynamic fluorescence quenching elements.ACS Sens. 2016 Apr 22;1(4):427-436. doi: 10.1021/acssensors.5b00252. Epub 2016 Feb 9. ACS Sens. 2016. PMID: 28944290 Free PMC article.
-
Molecular Probes for Imaging Fibrosis and Fibrogenesis.Chemistry. 2019 Jan 24;25(5):1128-1141. doi: 10.1002/chem.201801578. Epub 2018 Nov 21. Chemistry. 2019. PMID: 30014529 Free PMC article. Review.
-
Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging.Part Fibre Toxicol. 2010 Mar 3;7:3. doi: 10.1186/1743-8977-7-3. Part Fibre Toxicol. 2010. PMID: 20199661 Free PMC article. Review.
References
-
- Bayat H, Swaney JS, Ander AN, Dalton N, Kennedy BP, Hammond HK, Roth DM. Progressive heart failure after myocardial infarction in mice. Basic Res Cardiol. 2002;97:206–13. - PubMed
-
- Weisman HF, Bush DE, Mannisi JA, Weisfeldt ML, Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988;78:186–201. - PubMed
-
- Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–20. - PubMed
-
- Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol. 2002;87:547–55. - PubMed
-
- Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

