Potassium channels in the peripheral microcirculation
- PMID: 15804979
- PMCID: PMC1405752
- DOI: 10.1080/10739680590896072
Potassium channels in the peripheral microcirculation
Abstract
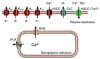
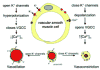
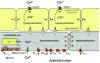
Vascular smooth muscle (VSM) cells, endothelial cells (EC), and pericytes that form the walls of vessels in the microcirculation express a diverse array of ion channels that play an important role in the function of these cells and the microcirculation in both health and disease. This brief review focuses on the K+ channels expressed in smooth muscle and endothelial cells in arterioles. Microvascular VSM cells express at least four different classes of K+ channels, including inward-rectifier K+ channels (Kin), ATP-sensitive K+ channels (KATP), voltage-gated K+ channels (Kv), and large conductance Ca2+-activated K+ channels (BKCa). VSM KIR participate in dilation induced by elevated extracellular K+ and may also be activated by C-type natriuretic peptide, a putative endothelium-derived hyperpolarizing factor (EDHF). Vasodilators acting through cAMP or cGMP signaling pathways in VSM may open KATP, Kv, and BKCa, causing membrane hyperpolarization and vasodilation. VSMBKc. may also be activated by epoxides of arachidonic acid (EETs) identified as EDHF in some systems. Conversely, vasoconstrictors may close KATP, Kv, and BKCa through protein kinase C, Rho-kinase, or c-Src pathways and contribute to VSM depolarization and vasoconstriction. At the same time Kv and BKCa act in a negative feedback manner to limit depolarization and prevent vasospasm. Microvascular EC express at least 5 classes of K+ channels, including small (sKCa) and intermediate(IKCa) conductance Ca2+-activated K+ channels, Kin, KATP, and Kv. Both sK and IK are opened by endothelium-dependent vasodilators that increase EC intracellular Ca2+ to cause membrane hyper-polarization that may be conducted through myoendothelial gap junctions to hyperpolarize and relax arteriolar VSM. KIR may serve to amplify sKCa- and IKCa-induced hyperpolarization and allow active transmission of hyperpolarization along EC through gap junctions. EC KIR channels may also be opened by elevated extracellular K+ and participate in K+-induced vasodilation. EC KATP channels may be activated by vasodilators as in VSM. Kv channels may provide a negative feedback mechanism to limit depolarization in some endothelial cells.
Figures

Similar articles
-
Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease.Clin Exp Pharmacol Physiol. 2004 Sep;31(9):641-9. doi: 10.1111/j.1440-1681.2004.04053.x. Clin Exp Pharmacol Physiol. 2004. PMID: 15479173 Review.
-
K+ channel modulation in arterial smooth muscle.Acta Physiol Scand. 1998 Dec;164(4):549-57. doi: 10.1046/j.1365-201X.1998.00433.x. Acta Physiol Scand. 1998. PMID: 9887977 Review.
-
Physiological roles of K+ channels in vascular smooth muscle cells.J Smooth Muscle Res. 2008 Apr;44(2):65-81. doi: 10.1540/jsmr.44.65. J Smooth Muscle Res. 2008. PMID: 18552454 Review.
-
Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth.Adv Pharmacol. 2017;78:89-144. doi: 10.1016/bs.apha.2016.07.001. Epub 2016 Aug 17. Adv Pharmacol. 2017. PMID: 28212804 Free PMC article. Review.
-
Calcium-Dependent Ion Channels and the Regulation of Arteriolar Myogenic Tone.Front Physiol. 2021 Nov 8;12:770450. doi: 10.3389/fphys.2021.770450. eCollection 2021. Front Physiol. 2021. PMID: 34819877 Free PMC article. Review.
Cited by
-
Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells.Drug Discov Today. 2012 Sep;17(17-18):974-87. doi: 10.1016/j.drudis.2012.04.002. Epub 2012 Apr 13. Drug Discov Today. 2012. PMID: 22521666 Free PMC article. Review.
-
ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans.J Physiol. 2012 Nov 1;590(21):5349-59. doi: 10.1113/jphysiol.2012.234245. Epub 2012 Jul 9. J Physiol. 2012. PMID: 22777673 Free PMC article. Clinical Trial.
-
KV1.3: a new therapeutic target to control vascular smooth muscle cell proliferation.Arterioscler Thromb Vasc Biol. 2010 Jun;30(6):1073-4. doi: 10.1161/ATVBAHA.110.206565. Arterioscler Thromb Vasc Biol. 2010. PMID: 20484702 Free PMC article. No abstract available.
-
Differential hyperpolarization to substance P and calcitonin gene-related peptide in smooth muscle versus endothelium of mouse mesenteric artery.Microcirculation. 2021 Nov;28(8):e12733. doi: 10.1111/micc.12733. Epub 2021 Oct 21. Microcirculation. 2021. PMID: 34633728 Free PMC article.
-
Methyl-Beta-Cyclodextrin Restores KIR Channel Function in Brain Endothelium of Female Alzheimer's Disease Mice.J Alzheimers Dis Rep. 2021 Sep 3;5(1):693-703. doi: 10.3233/ADR-210016. eCollection 2021. J Alzheimers Dis Rep. 2021. PMID: 34755043 Free PMC article.
References
-
- Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. Can J Physiol Pharmacol. 1994;72:47.
-
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1995;268:H926–H934. - PubMed
-
- Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275:H448–H459. - PubMed
-
- Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–H119. - PubMed
-
- Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

