CXCL9 inhibits eosinophil responses by a CCR3- and Rac2-dependent mechanism
- PMID: 15802529
- PMCID: PMC1895169
- DOI: 10.1182/blood-2005-02-0489
CXCL9 inhibits eosinophil responses by a CCR3- and Rac2-dependent mechanism
Abstract
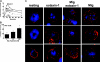
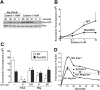
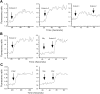
Recently, inhibitory cytokine pathways for leukocyte chemoattraction and activation have been identified, but there is little insight into the operational mechanisms except for models that rely on simple receptor antagonism. We have previously identified the existence of a murine eosinophil inhibitory pathway mediated by the CXC chemokine ligand 9 (CXCL9, Mig [monokine induced by interferon-gamma]) that impressively blocks eosinophil chemoattraction and function, but the mechanism has remained elusive. We now demonstrate that Mig's inhibitory action extends beyond receptor antagonism alone. Notably, in addition to inhibiting eotaxin-induced filamentous actin (F-actin) formation and chemoattraction, Mig potently blocks platelet activating factor (PAF)- and leukotriene B4 (LTB4)-induced responses. Remarkably, Mig-treated eosinophils display an abnormal F-actin assembly in the absence of agonist stimulation. Additionally, Mig pretreatment inhibits eotaxin-induced activation of the Rho-guanosine triphosphatase (GTPase) Rac, and Rac2-deficient eosinophils demonstrate an impaired transmigration and actin polymerization response to eotaxin stimulation. Furthermore, Mig was unable to inhibit eotaxin-induced responses in Rac2-deficient eosinophils. Finally, using CCR3 gene-targeted cells, Mig's inhibitory activity is demonstrated to be mediated by CC chemokine receptor 3 (CCR3). Thus, by altering agonist-induced signaling and abrogating cytoskeletal reorganization by a Rac2-dependent mechanism, Mig markedly inhibits eosinophil responses to diverse stimuli. These results establish evidence that distinct chemokines can use CCR3 to induce opposing signals in eosinophils.
Figures
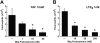
Similar articles
-
Negative regulation of eosinophil recruitment to the lung by the chemokine monokine induced by IFN-gamma (Mig, CXCL9).Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):1987-92. doi: 10.1073/pnas.0308544100. Epub 2004 Feb 9. Proc Natl Acad Sci U S A. 2004. PMID: 14769916 Free PMC article.
-
The ligands of CXC chemokine receptor 3, I-TAC, Mig, and IP10, are natural antagonists for CCR3.J Biol Chem. 2001 Feb 2;276(5):2986-91. doi: 10.1074/jbc.M005652200. Epub 2000 Nov 10. J Biol Chem. 2001. PMID: 11110785
-
Agonist activation of f-actin-mediated eosinophil shape change and mediator release is dependent on Rac2.Int Arch Allergy Immunol. 2011;156(2):137-47. doi: 10.1159/000322597. Epub 2011 May 16. Int Arch Allergy Immunol. 2011. PMID: 21576984 Free PMC article.
-
Regulation of chemokine receptor expression in eosinophils.Int Arch Allergy Immunol. 2001;125 Suppl 1:29-32. doi: 10.1159/000053849. Int Arch Allergy Immunol. 2001. PMID: 11408769 Review.
-
Eotaxin and asthma.Curr Opin Pharmacol. 2001 Jun;1(3):248-53. doi: 10.1016/s1471-4892(01)00044-3. Curr Opin Pharmacol. 2001. PMID: 11712747 Review.
Cited by
-
Aberrant inflammatory response to Streptococcus pyogenes in mice lacking myeloid differentiation factor 88.Am J Pathol. 2010 Feb;176(2):754-63. doi: 10.2353/ajpath.2010.090422. Epub 2009 Dec 17. Am J Pathol. 2010. PMID: 20019195 Free PMC article.
-
Rho and Rac, but not ROCK, are required for secretion of human and mouse eosinophil-associated RNases.Clin Exp Allergy. 2019 Feb;49(2):190-198. doi: 10.1111/cea.13292. Epub 2018 Nov 19. Clin Exp Allergy. 2019. PMID: 30295352 Free PMC article.
-
Up-regulation of chemokine C-C ligand 2 (CCL2) and C-X-C chemokine 8 (CXCL8) expression by monocytes in chronic idiopathic urticaria.Clin Exp Immunol. 2012 Jan;167(1):129-36. doi: 10.1111/j.1365-2249.2011.04485.x. Clin Exp Immunol. 2012. PMID: 22132892 Free PMC article.
-
Aiolos regulates eosinophil migration into tissues.Mucosal Immunol. 2021 Nov;14(6):1271-1281. doi: 10.1038/s41385-021-00416-4. Epub 2021 Aug 2. Mucosal Immunol. 2021. PMID: 34341502 Free PMC article.
-
Modulation of Chemokine Responses: Synergy and Cooperativity.Front Immunol. 2016 May 19;7:183. doi: 10.3389/fimmu.2016.00183. eCollection 2016. Front Immunol. 2016. PMID: 27242790 Free PMC article. Review.
References
-
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2: 108-115. - PubMed
-
- Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14: 129-135. - PubMed
-
- Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10: 259-264. - PubMed
-
- Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14: 195-200. - PubMed
-
- Blanpain C, Migeotte I, Lee B, et al. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94: 1899-1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

