Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles
- PMID: 15795308
- PMCID: PMC1069558
- DOI: 10.1128/JVI.79.8.5232-5237.2005
Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles
Abstract
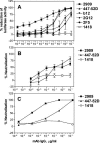
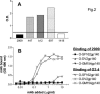
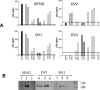
The selection of human monoclonal antibodies (MAbs) specific for human immunodeficiency virus (HIV) type 1 by binding assays may fail to identify Abs to quaternary epitopes on the intact virions. The HIV neutralization assay was used for the selection of human MAb 2909, which potently neutralizes SF162 and recognizes an epitope on the virus surface but not on soluble proteins. Three regions of gp120, the V2 and V3 loops and the CD4 binding domain, contribute to the epitope recognized by MAb 2909. The existence of such a unique MAb, which defines a complex epitope formed by a quaternary structure, suggests that there may be other new neutralizing HIV epitopes to target with vaccines.
Figures
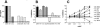
Similar articles
-
The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope.J Virol. 2004 Mar;78(5):2394-404. doi: 10.1128/jvi.78.5.2394-2404.2004. J Virol. 2004. PMID: 14963135 Free PMC article.
-
The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type 1 primary isolates.J Virol. 2005 Jun;79(11):6909-17. doi: 10.1128/JVI.79.11.6909-6917.2005. J Virol. 2005. PMID: 15890930 Free PMC article.
-
Type-specific epitopes targeted by monoclonal antibodies with exceptionally potent neutralizing activities for selected strains of human immunodeficiency virus type 1 map to a common region of the V2 domain of gp120 and differ only at single positions from the clade B consensus sequence.J Virol. 2007 Feb;81(3):1424-32. doi: 10.1128/JVI.02054-06. Epub 2006 Nov 22. J Virol. 2007. PMID: 17121806 Free PMC article.
-
Human monoclonal antibody 2909 binds to pseudovirions expressing trimers but not monomeric HIV-1 envelope proteins.Hum Antibodies. 2009;18(1-2):35-40. doi: 10.3233/HAB-2009-0200. Hum Antibodies. 2009. PMID: 19478397 Free PMC article.
-
A novel human antibody against human immunodeficiency virus type 1 gp120 is V1, V2, and V3 loop dependent and helps delimit the epitope of the broadly neutralizing antibody immunoglobulin G1 b12.J Virol. 2003 Jun;77(12):6965-78. doi: 10.1128/jvi.77.12.6965-6978.2003. J Virol. 2003. PMID: 12768015 Free PMC article.
Cited by
-
The V1V2 Region of HIV-1 gp120 Forms a Five-Stranded Beta Barrel.J Virol. 2015 Aug;89(15):8003-10. doi: 10.1128/JVI.00754-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018158 Free PMC article.
-
Conserved structural elements in the V3 crown of HIV-1 gp120.Nat Struct Mol Biol. 2010 Aug;17(8):955-61. doi: 10.1038/nsmb.1861. Epub 2010 Jul 11. Nat Struct Mol Biol. 2010. PMID: 20622876
-
Interaction of the gp120 V1V2 loop with a neighboring gp120 unit shields the HIV envelope trimer against cross-neutralizing antibodies.J Exp Med. 2011 Jul 4;208(7):1419-33. doi: 10.1084/jem.20110196. Epub 2011 Jun 6. J Exp Med. 2011. PMID: 21646396 Free PMC article.
-
Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C.J Virol. 2008 Dec;82(23):11651-68. doi: 10.1128/JVI.01762-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815292 Free PMC article.
-
HIV-1 variable loop 2 and its importance in HIV-1 infection and vaccine development.Curr HIV Res. 2013 Jul;11(5):427-38. doi: 10.2174/1570162x113116660064. Curr HIV Res. 2013. PMID: 24191938 Free PMC article.
References
-
- Andrus, L., A. M. Prince, I. Bernal, P. McCormack, D. H. Lee, M. K. Gorny, and S. Zolla-Pazner. 1998. Passive immunization with a human immunodeficiency virus type-1 neutralizing monoclonal antibody in Hu-PBL-SCID mice: isolation of a neutralization escape variant. J. Infect. Dis. 177:889-897. - PubMed
-
- Bandres, J. C., Q. F. Wang, J. O'Leary, F. Baleaux, A. Amara, J. A. Hoxie, S. Zolla-Pazner, and M. K. Gorny. 1998. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J. Virol. 72:2500-2504. - PMC - PubMed
-
- Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, and H. Katinger. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359-369. - PubMed
-
- Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

