Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation
- PMID: 15784587
- PMCID: PMC1087423
- DOI: 10.1128/IAI.73.4.2411-2423.2005
Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation
Abstract
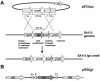
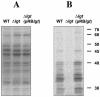
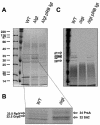
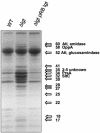
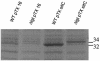
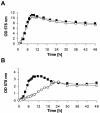
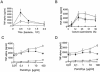
A lipoprotein diacylglyceryl transferase (lgt) deletion mutant of Staphylococcus aureus SA113 was constructed. The lipoprotein and prelipoprotein expression, the growth behavior, and the ability of the mutant to elicit an immune response in various host cells were studied. In the wild type, the majority of [14C]palmitate-labeled lipoproteins were located in the membrane fraction, although some lipoproteins were also present on the cell surface and in the culture supernatant. The lgt mutant completely lacked palmitate-labeled lipoproteins and released high amounts of some unmodified prelipoproteins, e.g., the oligopeptide-binding protein OppA, the peptidyl-prolyl cis-trans isomerase PrsA, and the staphylococcal iron transporter SitC, into the culture supernatant. The growth of the lgt mutant was hardly affected in rich medium but was retarded under nutrient limitation. The lgt mutant and its crude lysate induced much fewer proinflammatory cytokines and chemokines in human monocytic (MonoMac6), epithelial (pulmonary A549), and endothelial (human umbilical vein endothelial) cells than the wild type. However, in whole blood samples, the culture supernatant of the lgt mutant was equal or even superior to the wild-type supernatant in tumor necrosis factor alpha induction. Lipoprotein fractionation experiments provided evidence that a small proportion of the mature lipoproteins are released by the S. aureus wild type despite the lipid anchor and are trapped in part by the cell wall, thereby exposing the immune-activating lipid structure on the cell surface. Bacterial lipoproteins appear to be essential for a complete immune stimulation by gram-positive bacteria.
Figures
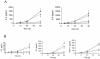

Similar articles
-
The role of phagocytosis in IL-8 production by human monocytes in response to lipoproteins on Staphylococcus aureus.Biochem Biophys Res Commun. 2011 Mar 18;406(3):449-53. doi: 10.1016/j.bbrc.2011.02.069. Epub 2011 Feb 17. Biochem Biophys Res Commun. 2011. PMID: 21333631
-
Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes.J Bacteriol. 2007 Jan;189(2):313-24. doi: 10.1128/JB.00976-06. Epub 2006 Oct 13. J Bacteriol. 2007. PMID: 17041050 Free PMC article.
-
Role of prolipoprotein diacylglyceryl transferase (Lgt) and lipoprotein-specific signal peptidase II (LspA) in localization and physiological function of lipoprotein MsmE in Streptococcus mutans.Oral Microbiol Immunol. 2008 Dec;23(6):515-9. doi: 10.1111/j.1399-302X.2008.00455.x. Oral Microbiol Immunol. 2008. PMID: 18954360
-
Staphylococcal lipoproteins and their role in bacterial survival in mice.Int J Med Microbiol. 2010 Feb;300(2-3):155-60. doi: 10.1016/j.ijmm.2009.08.018. Epub 2009 Oct 4. Int J Med Microbiol. 2010. PMID: 19805005 Review.
-
Lipoproteins in bacteria: structures and biosynthetic pathways.FEBS J. 2012 Dec;279(23):4247-68. doi: 10.1111/febs.12041. Epub 2012 Nov 7. FEBS J. 2012. PMID: 23094979 Review.
Cited by
-
Lipoproteins in Gram-Positive Bacteria: Abundance, Function, Fitness.Front Microbiol. 2020 Sep 18;11:582582. doi: 10.3389/fmicb.2020.582582. eCollection 2020. Front Microbiol. 2020. PMID: 33042100 Free PMC article. Review.
-
NisI Maturation and Its Influence on Nisin Resistance in Lactococcus lactis.Appl Environ Microbiol. 2020 Sep 17;86(19):e01306-20. doi: 10.1128/AEM.01306-20. Print 2020 Sep 17. Appl Environ Microbiol. 2020. PMID: 32709730 Free PMC article.
-
Lipoprotein N-Acylation in Staphylococcus aureus Is Catalyzed by a Two-Component Acyl Transferase System.mBio. 2020 Jul 28;11(4):e01619-20. doi: 10.1128/mBio.01619-20. mBio. 2020. PMID: 32723923 Free PMC article.
-
Proteome analysis and serological characterization of surface-exposed proteins of Rickettsia heilongjiangensis.PLoS One. 2013 Jul 23;8(7):e70440. doi: 10.1371/journal.pone.0070440. Print 2013. PLoS One. 2013. PMID: 23894656 Free PMC article.
-
The νSaα Specific Lipoprotein Like Cluster (lpl) of S. aureus USA300 Contributes to Immune Stimulation and Invasion in Human Cells.PLoS Pathog. 2015 Jun 17;11(6):e1004984. doi: 10.1371/journal.ppat.1004984. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26083414 Free PMC article.
References
-
- Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. - PubMed
-
- Arnott, D., K. L. O'Connell, K. L. King, and J. T. Stults. 1998. An integrated approach to proteome analysis: identification of proteins associated with cardiac hypertrophy. Anal. Biochem. 258:1-18. - PubMed
-
- Brehmer, D., C. Gassler, W. Rist, M. P. Mayer, and B. Bukau. 2004. Influence of GrpE on DnaK-substrate interactions. J. Biol. Chem. 279:27957-27964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

