Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins
- PMID: 15769892
- PMCID: PMC1894991
- DOI: 10.1182/blood-2004-11-4274
Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins
Abstract
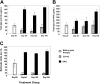
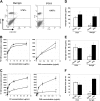
Up to 30% of patients with hemophilia A given therapeutic factor VIII (fVIII) can make inhibitory antibodies, the majority of which are reactive with its C2 and A2 domains. We have previously demonstrated that antigen-specific tolerance to several antigens can be induced by lipopolysaccharide (LPS)-activated B-cell blasts transduced with immunoglobulin (IgG)-antigen fusion constructs. To apply this system to hemophilia A inhibitor formation, we created retroviral vectors expressing fVIII amino acids S2173-Y2332 (C2 domain) and S373-R740 (A2 domain) in frame with an IgG heavy chain backbone. These vectors were transduced into B-cell blasts to induce tolerance in both naive and fVIII-primed hemophilic (E16 fVIII(-/-)) mice. Thus, treatment of E16 fVIII(-/-) mice with B cells expressing fVIII C2 and A2 domains led to tolerance in terms of specific humoral response (including inhibitory antibody titers) and cellular responses to fVIII and its C2 or A2 domains. Moreover, a significant reduction in immune responses to fVIII could be achieved in immunized hemophilic mice with existing anti-fVIII titers. This hyporesponsive state persisted for at least 2 months and withstood additional challenge with fVIII. Further experiments, in which mice were treated with a depleting monoclonal anti-CD25, suggested that a regulatory T cell may be required for the tolerogenic effect of transduced B cells. These findings demonstrate that B-cell presentation of fVIII domains on an Ig backbone specifically prevents or decreases existing antibodies in hemophilia A mice.
Figures



Similar articles
-
Immune tolerance induced by platelet-targeted factor VIII gene therapy in hemophilia A mice is CD4 T cell mediated.J Thromb Haemost. 2017 Oct;15(10):1994-2004. doi: 10.1111/jth.13800. Epub 2017 Sep 11. J Thromb Haemost. 2017. PMID: 28799202 Free PMC article.
-
Targeting Antigen-Specific B Cells Using Antigen-Expressing Transduced Regulatory T Cells.J Immunol. 2018 Sep 1;201(5):1434-1441. doi: 10.4049/jimmunol.1701800. Epub 2018 Jul 18. J Immunol. 2018. PMID: 30021767 Free PMC article.
-
Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy.PLoS One. 2012;7(5):e37671. doi: 10.1371/journal.pone.0037671. Epub 2012 May 24. PLoS One. 2012. PMID: 22655063 Free PMC article.
-
Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives.Blood Coagul Fibrinolysis. 2004 Mar;15(2):109-24. doi: 10.1097/00001721-200403000-00001. Blood Coagul Fibrinolysis. 2004. PMID: 15090997 Review.
-
The molecular mechanisms of immunomodulation and tolerance induction to factor VIII.J Thromb Haemost. 2009 Sep;7(9):1446-56. doi: 10.1111/j.1538-7836.2009.03538.x. Epub 2009 Jul 6. J Thromb Haemost. 2009. PMID: 19583822 Review.
Cited by
-
Animal models of hemophilia.Prog Mol Biol Transl Sci. 2012;105:151-209. doi: 10.1016/B978-0-12-394596-9.00006-8. Prog Mol Biol Transl Sci. 2012. PMID: 22137432 Free PMC article. Review.
-
Tolerance to MHC class II disparate allografts through genetic modification of bone marrow.Gene Ther. 2013 May;20(5):478-86. doi: 10.1038/gt.2012.57. Epub 2012 Jul 26. Gene Ther. 2013. PMID: 22833118 Free PMC article.
-
Factor VIII inhibitors: risk factors and methods for prevention and immune modulation.Clin Rev Allergy Immunol. 2009 Oct;37(2):114-24. doi: 10.1007/s12016-009-8122-5. Clin Rev Allergy Immunol. 2009. PMID: 19199081 Review.
-
A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells.Blood. 2009 Jul 16;114(3):677-85. doi: 10.1182/blood-2009-03-202267. Epub 2009 May 20. Blood. 2009. PMID: 19458355 Free PMC article.
-
Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells.Blood. 2014 Sep 4;124(10):1659-68. doi: 10.1182/blood-2013-10-528737. Epub 2014 May 13. Blood. 2014. PMID: 24825864 Free PMC article.
References
-
- Jacquemin MG, Saint-Remy JM. FVIII alloantibodies in hemophilia. Curr Opin Hematol. 2004;11: 146-150. - PubMed
-
- Villard S, Lacroix-Desmazes S, Kieber-Emmons T, et al. Peptide decoys selected by phage display block in vitro and in vivo activity of a human anti-fVIII inhibitor. Blood. 2003;102: 949-952. - PubMed
-
- Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P. Reduction of the antigenicity of fVIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine fVIII molecules. Blood. 2000;15; 95:564-568. - PubMed
-
- Ananyeva NM, Lacroix-Desmazes S, Hauser CA, et al. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004;15: 109-124. - PubMed
-
- Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95: 1324-1329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

