Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability
- PMID: 15767571
- PMCID: PMC555497
- DOI: 10.1073/pnas.0407822102
Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability
Abstract
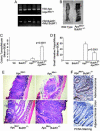
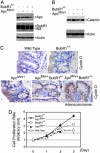
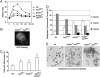
Faithful chromosome segregation is essential for the maintenance of genetic stability during cell division and it is at least partly monitored by the spindle checkpoint, a surveillance mechanism preventing the cell from prematurely entering anaphase. The adenomatous polyposis coli (Apc) gene also plays an important role in regulating genomic stability, as mutations of Apc cause aneuploidy. Here we show that whereas Apc(Min)(/+) mice developed many adenomatous polyps, mostly in the small intestine, by 3 mo of age; BubR1(+/-)Apc(Min)(/+) compound mutant mice developed 10 times more colonic tumors than Apc(Min)(/+) mice. The colonic tumors in BubR1(+/-)Apc(Min)(/+) mice were in higher grades than those observed in Apc(Min)(/+) mice. Consistently, BubR1(+/-)Apc(Min)(/+) murine embryonic fibroblasts (MEFs) contained more beta-catenin and proliferated at a faster rate than WT or BubR1(+/-) MEFs. Moreover, BubR1(+/-)Apc(Min)(/+) MEFs slipped through mitosis in the presence of nocodazole and exhibited a higher rate of genomic instability than that of WT or BubR1(+/-) or Apc(Min)(/+) MEFs, accompanied by premature separation of sister chromatids. Together, our studies suggest that BubR1 and Apc functionally interact in regulating metaphase-anaphase transition, deregulation of which may play a key role in genomic instability and development and progression of colorectal cancer.
Figures

Similar articles
-
Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/- compound mutant mice.Nat Genet. 2003 Dec;35(4):323-30. doi: 10.1038/ng1265. Epub 2003 Nov 16. Nat Genet. 2003. PMID: 14625550
-
APC inactivation associates with abnormal mitosis completion and concomitant BUB1B/MAD2L1 up-regulation.Gastroenterology. 2007 Jun;132(7):2448-58. doi: 10.1053/j.gastro.2007.03.027. Epub 2007 Mar 19. Gastroenterology. 2007. PMID: 17570218
-
p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase.Oncogene. 2000 Sep 21;19(40):4557-62. doi: 10.1038/sj.onc.1203803. Oncogene. 2000. PMID: 11030144
-
Enhanced genomic instabilities caused by deregulated microtubule dynamics and chromosome segregation: a perspective from genetic studies in mice.Carcinogenesis. 2009 Sep;30(9):1469-74. doi: 10.1093/carcin/bgp081. Epub 2009 Apr 16. Carcinogenesis. 2009. PMID: 19372138 Free PMC article. Review.
-
BubR1 kinase: protection against aneuploidy and premature aging.Trends Mol Med. 2015 Jun;21(6):364-72. doi: 10.1016/j.molmed.2015.04.003. Epub 2015 May 8. Trends Mol Med. 2015. PMID: 25964054 Review.
Cited by
-
Physiological relevance of post-translational regulation of the spindle assembly checkpoint protein BubR1.Cell Biosci. 2021 Apr 23;11(1):76. doi: 10.1186/s13578-021-00589-2. Cell Biosci. 2021. PMID: 33892776 Free PMC article. Review.
-
High expression of BUBR1 is one of the factors for inducing DNA aneuploidy and progression in gastric cancer.Cancer Sci. 2010 Mar;101(3):639-45. doi: 10.1111/j.1349-7006.2009.01457.x. Epub 2009 Dec 4. Cancer Sci. 2010. PMID: 20132214 Free PMC article.
-
Lemongrass Extract Possesses Potent Anticancer Activity Against Human Colon Cancers, Inhibits Tumorigenesis, Enhances Efficacy of FOLFOX, and Reduces Its Adverse Effects.Integr Cancer Ther. 2019 Jan-Dec;18:1534735419889150. doi: 10.1177/1534735419889150. Integr Cancer Ther. 2019. PMID: 31845598 Free PMC article.
-
Deregulated Aurora-B induced tetraploidy promotes tumorigenesis.FASEB J. 2009 Aug;23(8):2741-8. doi: 10.1096/fj.09-130963. Epub 2009 Mar 30. FASEB J. 2009. PMID: 19332642 Free PMC article.
-
Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis.Nat Cell Biol. 2016 Jan;18(1):100-10. doi: 10.1038/ncb3270. Epub 2015 Nov 23. Nat Cell Biol. 2016. PMID: 26595384
References
-
- Storchova, Z. & Pellman, D. (2004) Nat. Rev. Mol. Cell Biol. 5, 45–54. - PubMed
-
- Motoyama, N. & Naka, K. (2004) Curr. Opin. Genet. Dev. 14, 11–16. - PubMed
-
- Doxsey, S. (2002) Mol. Cell 10, 439–440. - PubMed
-
- Lengauer, C., Kinzler, K. W. & Vogelstein, B. (1997) Nature 386, 623–627. - PubMed
-
- Rajagopalan, H., Nowak, M. A., Vogelstein, B. & Lengauer, C. (2003) Nat. Rev. Cancer 3, 695–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

