Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation
- PMID: 15767448
- PMCID: PMC1061522
- DOI: 10.1128/JVI.79.7.4479-4491.2005
Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation
Abstract
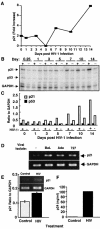
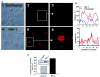
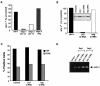
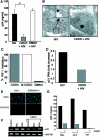
In contrast to CD4+ T cells, human immunodeficiency virus type 1 (HIV-1)-infected macrophages typically resist cell death, support viral replication, and consequently, may facilitate HIV-1 transmission. To elucidate how the virus commandeers the macrophage's intracellular machinery for its benefit, we analyzed HIV-1-infected human macrophages for virus-induced gene transcription by using multiple parameters, including cDNA expression arrays. HIV-1 infection induced the transcriptional regulation of genes associated with host defense, signal transduction, apoptosis, and the cell cycle, among which the cyclin-dependent kinase inhibitor 1A (CDKN1A/p21) gene was the most prominent. p21 mRNA and protein expression followed a bimodal pattern which was initially evident during the early stages of infection, and maximum levels occurred concomitant with active HIV-1 replication. Mechanistically, viral protein R (Vpr) independently regulates p21 expression, consistent with the reduced viral replication and lack of p21 upregulation by a Vpr-negative virus. Moreover, the treatment of macrophages with p21 antisense oligonucleotides or small interfering RNAs reduced HIV-1 infection. In addition, the synthetic triterpenoid and peroxisome proliferator-activated receptor gamma ligand, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), which is known to influence p21 expression, suppressed viral replication. These data implicate p21 as a pivotal macrophage facilitator of the viral life cycle. Moreover, regulators of p21, such as CDDO, may provide an interventional approach to modulate HIV-1 replication.
Figures



Similar articles
-
Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo.J Virol. 2006 Nov;80(21):10407-18. doi: 10.1128/JVI.01212-06. Epub 2006 Sep 6. J Virol. 2006. PMID: 16956949 Free PMC article.
-
Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells.Cancer Res. 2003 Sep 15;63(18):5926-39. Cancer Res. 2003. PMID: 14522919
-
Phenotype of HIV-1 lacking a functional nuclear localization signal in matrix protein of gag and Vpr is comparable to wild-type HIV-1 in primary macrophages.Virology. 1999 Jan 20;253(2):170-80. doi: 10.1006/viro.1998.9482. Virology. 1999. PMID: 9918876
-
Effect of HIV-1 Vpr on cell cycle regulators.DNA Cell Biol. 2004 Apr;23(4):249-60. doi: 10.1089/104454904773819833. DNA Cell Biol. 2004. PMID: 15142382 Review.
-
Insights into the biology of HIV-1 viral protein R.DNA Cell Biol. 2002 Sep;21(9):679-88. doi: 10.1089/104454902760330228. DNA Cell Biol. 2002. PMID: 12396611 Review.
Cited by
-
Transcriptome Profiling of Human Monocyte-Derived Macrophages Upon CCL2 Neutralization Reveals an Association Between Activation of Innate Immune Pathways and Restriction of HIV-1 Gene Expression.Front Immunol. 2020 Sep 18;11:2129. doi: 10.3389/fimmu.2020.02129. eCollection 2020. Front Immunol. 2020. PMID: 33072075 Free PMC article.
-
HIV-1 activates macrophages independent of Toll-like receptors.PLoS One. 2008;3(12):e3664. doi: 10.1371/journal.pone.0003664. Epub 2008 Dec 2. PLoS One. 2008. PMID: 19048100 Free PMC article.
-
Transcriptome analysis of primary monocytes from HIV-positive patients with differential responses to antiretroviral therapy.Virol J. 2013 Dec 27;10:361. doi: 10.1186/1743-422X-10-361. Virol J. 2013. PMID: 24370116 Free PMC article.
-
HIV-1 persistence in the CNS: Mechanisms of latency, pathogenesis and an update on eradication strategies.Virus Res. 2021 Oct 2;303:198523. doi: 10.1016/j.virusres.2021.198523. Epub 2021 Jul 24. Virus Res. 2021. PMID: 34314771 Free PMC article. Review.
-
Macroautophagy Regulation during HIV-1 Infection of CD4+ T Cells and Macrophages.Front Immunol. 2012 May 7;3:97. doi: 10.3389/fimmu.2012.00097. eCollection 2012. Front Immunol. 2012. PMID: 22586428 Free PMC article.
References
-
- Antonsson, B., and J. C. Martinou. 2000. The Bcl-2 protein family. Exp. Cell Res. 256:50-57. - PubMed
-
- Arany, I., A. Yen, and S. K. Tyring. 1997. p53, WAF1/CIP1 and mdm2 expression in skin lesions associated with human papillomavirus and human immunodeficiency virus. Anticancer Res. 17:1281-1285. - PubMed
-
- Arber, S., F. A. Barbayannis, H. Hanser, C. Schneider, C. A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805-809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

