Human Immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases
- PMID: 15767427
- PMCID: PMC1061575
- DOI: 10.1128/JVI.79.7.4257-4269.2005
Human Immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases
Abstract
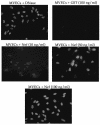
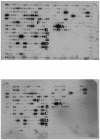
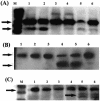
The lentiviral protein Nef plays a major role in the pathogenesis of human immunodeficiency virus type I (HIV-1) infection. Although the exact mechanisms of its actions are not fully understood, Nef has been shown to be essential for the maintenance of high-titer viral replication and disease pathogenesis in in vivo models of simian immunodeficiency virus infection of monkeys. Nef has also been suggested to play a pivotal role in the depletion of T cells by promoting apoptosis in bystander cells. In this context, we investigated the ability of extracellular and endogenously expressed HIV-1 Nef to induce apoptosis in primary human brain microvascular endothelial cells (MVECs). Human brain MVECs were exposed to baculovirus-expressed HIV-1 Nef protein, an HIV-1-based vector expressing Nef, spleen necrosis virus (SNV)-Nef virus (i.e., SNV vector expressing HIV-1 Nef as a transgene), and the HIV-1 strain ADA and its Nef deletion mutant, ADADeltaNef. We observed that ADA Nef, the HIV-1 vector expressing Nef, and SNV-Nef were able to induce apoptosis in a dose-dependent manner. The mutant virus with a deletion in Nef was able to induce apoptosis in MVECs to modest levels, but the effects were not as pronounced as with the wild-type HIV-1 strain, ADA, the HIV-1-based vector expressing Nef, or SNV-Nef viruses. We also demonstrated that relatively high concentrations of exogenous HIV-1 Nef protein were able to induce apoptosis in MVECs. Gene microarray analyses showed increases in the expression of several specific proapoptotic genes. Western blot analyses revealed that the various caspases involved with Nef-induced apoptosis are processed into cleavage products, which occur only during programmed cell death. The results of this study demonstrate that Nef likely contributes to the neuroinvasion and neuropathogenesis of HIV-1, through its effects on select cellular processes, including various apoptotic cascades.
Figures






Similar articles
-
Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells.Virology. 2002 Dec 20;304(2):222-34. doi: 10.1006/viro.2002.1666. Virology. 2002. PMID: 12504564
-
Human endothelial cells enhance human immunodeficiency virus type 1 replication in CD4+ T cells in a Nef-dependent manner in vitro and in vivo.J Virol. 2005 Jan;79(1):264-76. doi: 10.1128/JVI.79.1.264-276.2005. J Virol. 2005. PMID: 15596822 Free PMC article.
-
The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages.J Exp Med. 1994 Jan 1;179(1):101-13. doi: 10.1084/jem.179.1.101. J Exp Med. 1994. PMID: 8270859 Free PMC article.
-
Could Nef and Vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells?Biochem Biophys Res Commun. 2000 Jan 27;267(3):677-85. doi: 10.1006/bbrc.1999.1708. Biochem Biophys Res Commun. 2000. PMID: 10673351 Review.
-
Human immunodeficiency virus (HIV) type-1, HIV-2 and simian immunodeficiency virus Nef proteins.Mol Aspects Med. 2010 Oct;31(5):418-33. doi: 10.1016/j.mam.2010.05.003. Epub 2010 Jun 4. Mol Aspects Med. 2010. PMID: 20594957 Review.
Cited by
-
HIV Nef-mediated Ubiquitination of BCL2: Implications in Autophagy and Apoptosis.Front Immunol. 2021 May 6;12:682624. doi: 10.3389/fimmu.2021.682624. eCollection 2021. Front Immunol. 2021. PMID: 34025682 Free PMC article.
-
HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells.J Surg Res. 2009 Oct;156(2):257-64. doi: 10.1016/j.jss.2009.02.005. Epub 2009 Mar 4. J Surg Res. 2009. PMID: 19540523 Free PMC article.
-
Magnetic nanotherapeutics for dysregulated synaptic plasticity during neuroAIDS and drug abuse.Mol Brain. 2016 May 23;9(1):57. doi: 10.1186/s13041-016-0236-0. Mol Brain. 2016. PMID: 27216740 Free PMC article. Review.
-
Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update.Front Cell Neurosci. 2015 Jun 10;9:212. doi: 10.3389/fncel.2015.00212. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26113810 Free PMC article. Review.
-
HIV Nef Expression Down-modulated GFAP Expression and Altered Glutamate Uptake and Release and Proliferation in Astrocytes.Aging Dis. 2023 Feb 1;14(1):152-169. doi: 10.14336/AD.2022.0712. eCollection 2023 Feb 1. Aging Dis. 2023. PMID: 36818564 Free PMC article.
References
-
- Acheampong, E., M. Mukhtar, Z. Parveen, N. Ngoubilly, N. Ahmad, C. Patel, and R. J. Pomerantz. 2002. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology 304:222-234. - PubMed
-
- Adachi, K., X. Li, H. Kimura, H. Amemiya, and S. Suzuki. 2001. HIV-1 Nef protein prolonged recipient survival in rat liver allografting. Transplant Proc. 33:284. - PubMed
-
- Adle-Biassette, H., Y. Levy, M. Colombel, F. Poron, S. Natchev, C. Keohane, and F. Gray. 1995. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 21:218-227. - PubMed
-
- Alimonti, J. B., T. B. Ball, and K. R. Fowke. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649-1661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

