Unchain my heart: the scientific foundations of cardiac repair
- PMID: 15765139
- PMCID: PMC1052009
- DOI: 10.1172/JCI24283
Unchain my heart: the scientific foundations of cardiac repair
Abstract
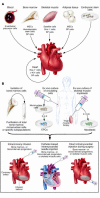
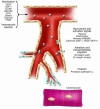
In humans, the biological limitations to cardiac regenerative growth create both a clinical imperative--to offset cell death in acute ischemic injury and chronic heart failure--and a clinical opportunity; that is, for using cells, genes, and proteins to rescue cardiac muscle cell number or in other ways promote more efficacious cardiac repair. Recent experimental studies and early-phase clinical trials lend credence to the visionary goal of enhancing cardiac repair as an achievable therapeutic target.
Figures

Similar articles
-
SDF-1α as a therapeutic stem cell homing factor in myocardial infarction.Pharmacol Ther. 2011 Jan;129(1):97-108. doi: 10.1016/j.pharmthera.2010.09.011. Epub 2010 Oct 20. Pharmacol Ther. 2011. PMID: 20965212 Review.
-
Cardiac repair by stem cells.Cell Death Differ. 2007 Jul;14(7):1258-61. doi: 10.1038/sj.cdd.4402146. Epub 2007 Apr 13. Cell Death Differ. 2007. PMID: 17431420 Review. No abstract available.
-
Restoration of cardiac function with progenitor cells.Novartis Found Symp. 2006;274:214-23; discussion 223-7, 272-6. Novartis Found Symp. 2006. PMID: 17019814 Review.
-
Lost and found: cardiac stem cell therapy revisited.J Clin Invest. 2006 Jul;116(7):1838-40. doi: 10.1172/JCI29050. J Clin Invest. 2006. PMID: 16823485 Free PMC article.
-
Cells, scaffolds, and molecules for myocardial tissue engineering.Pharmacol Ther. 2005 Feb;105(2):151-63. doi: 10.1016/j.pharmthera.2004.10.003. Epub 2004 Dec 8. Pharmacol Ther. 2005. PMID: 15670624 Review.
Cited by
-
Heart valve tissue engineering: concepts, approaches, progress, and challenges.Ann Biomed Eng. 2006 Dec;34(12):1799-819. doi: 10.1007/s10439-006-9163-z. Epub 2006 Oct 12. Ann Biomed Eng. 2006. PMID: 17053986 Free PMC article. Review.
-
No evidence of myocardial restoration following transplantation of mononuclear bone marrow cells in coronary bypass grafting surgery patients based upon cardiac SPECT and 18F-PET.BMC Med Imaging. 2006 Jul 14;6:7. doi: 10.1186/1471-2342-6-7. BMC Med Imaging. 2006. PMID: 16842625 Free PMC article.
-
Stem cell therapy for electrophysiological disorders.Curr Cardiol Rep. 2013 Oct;15(10):408. doi: 10.1007/s11886-013-0408-9. Curr Cardiol Rep. 2013. PMID: 23955788 Review.
-
Autologous cardiomyotissue implantation promotes myocardial regeneration, decreases infarct size, and improves left ventricular function.Circulation. 2011 Jan 4;123(1):62-9. doi: 10.1161/CIRCULATIONAHA.108.832469. Epub 2010 Dec 20. Circulation. 2011. PMID: 21173354 Free PMC article.
-
Cell therapy generates a favourable chemokine gradient for stem cell recruitment into the infarcted heart in rabbits.Eur J Heart Fail. 2009 Mar;11(3):238-45. doi: 10.1093/eurjhf/hfn035. Epub 2009 Jan 12. Eur J Heart Fail. 2009. PMID: 19147447 Free PMC article.
References
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. - PubMed
-
- Bettencourt-Dias M, Mittnacht S, Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J. Cell Sci. 2003;116:4001–4009. - PubMed
-
- Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 2005;96:110–118. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

