Ca2+ regulation in the absence of the iplA gene product in Dictyostelium discoideum
- PMID: 15760480
- PMCID: PMC555532
- DOI: 10.1186/1471-2121-6-13
Ca2+ regulation in the absence of the iplA gene product in Dictyostelium discoideum
Abstract
Background: Stimulation of Dictyostelium discoideum with cAMP evokes an elevation of the cytosolic free Ca2+ concentration ([Ca2+]i). The [Ca2+]i-change is composed of liberation of stored Ca2+ and extracellular Ca2+-entry. The significance of the [Ca2+]i-transient for chemotaxis is under debate. Abolition of chemotactic orientation and migration by Ca2+-buffers in the cytosol indicates that a [Ca2+]i-increase is required for chemotaxis. Yet, the iplA- mutant disrupted in a gene bearing similarity to IP3-receptors of higher eukaryotes aggregates despite the absence of a cAMP-induced [Ca2+]i-transient which favours the view that [Ca2+]i-changes are insignificant for chemotaxis.
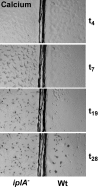
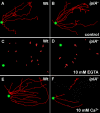
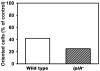
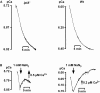
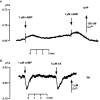
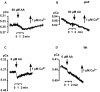
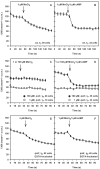
Results: We investigated Ca2+-fluxes and the effect of their disturbance on chemotaxis and development of iplA- cells. Differentiation was altered as compared to wild type amoebae and sensitive towards manipulation of the level of stored Ca2+. Chemotaxis was impaired when [Ca2+]i-transients were suppressed by the presence of a Ca2+-chelator in the cytosol of the cells. Analysis of ion fluxes revealed that capacitative Ca2+-entry was fully operative in the mutant. In suspensions of intact and permeabilized cells cAMP elicited extracellular Ca2+-influx and liberation of stored Ca2+, respectively, yet to a lesser extent than in wild type. In suspensions of partially purified storage vesicles ATP-induced Ca2+-uptake and Ca2+-release activated by fatty acids or Ca2+-ATPase inhibitors were similar to wild type. Mn2+-quenching of fura2 fluorescence allows to study Ca2+-influx indirectly and revealed that the responsiveness of mutant cells was shifted to higher concentrations: roughly 100 times more Mn2+ was necessary to observe agonist-induced Mn2+-influx. cAMP evoked a [Ca2+]i-elevation when stores were strongly loaded with Ca2+, again with a similar shift in sensitivity in the mutant. In addition, basal [Ca2+]i was significantly lower in iplA- than in wild type amoebae.
Conclusion: These results support the view that [Ca2+]i-transients are essential for chemotaxis and differentiation. Moreover, capacitative and agonist-activated ion fluxes are regulated by separate pathways that are mediated either by two types of channels in the plasma membrane or by distinct mechanisms coupling Ca2+-release from stores to Ca2+-entry in Dictyostelium. The iplA- strain retains the capacitative Ca2+-entry pathway and an impaired agonist-activated pathway that operates with reduced efficiency or at higher ionic pressure.
Figures



Similar articles
-
cAMP controls cytosolic Ca2+ levels in Dictyostelium discoideum.BMC Cell Biol. 2005 Mar 7;6(1):12. doi: 10.1186/1471-2121-6-12. BMC Cell Biol. 2005. PMID: 15752425 Free PMC article.
-
Cytosolic [Ca2+] transients in dictyostelium discoideum depend on the filling state of internal stores and on an active sarco/endoplasmic reticulum calcium ATPase (SERCA) Ca2+ pump.J Biol Chem. 2004 Apr 30;279(18):18407-14. doi: 10.1074/jbc.M307096200. Epub 2004 Feb 18. J Biol Chem. 2004. PMID: 14973132
-
Fatty acids induce release of Ca2+ from acidosomal stores and activate capacitative Ca2+ entry in Dictyostelium discoideum.Biochem J. 1998 Jun 1;332 ( Pt 2)(Pt 2):541-8. doi: 10.1042/bj3320541. Biochem J. 1998. PMID: 9601085 Free PMC article.
-
On the role of calcium during chemotactic signalling and differentiation of the cellular slime mould Dictyostelium discoideum.Int J Dev Biol. 1996 Feb;40(1):135-9. Int J Dev Biol. 1996. PMID: 8735922 Review.
-
Transduction of the chemotactic cAMP signal across the plasma membrane of Dictyostelium cells.Experientia. 1995 Dec 18;51(12):1144-54. doi: 10.1007/BF01944732. Experientia. 1995. PMID: 8536802 Review.
Cited by
-
Subversion of Phytomyxae Cell Communication With Surrounding Environment to Control Soilborne Diseases; A Case Study of Cytosolic Ca2+ Signal Disruption in Zoospores of Spongospora subterranea.Front Microbiol. 2022 Mar 1;13:754225. doi: 10.3389/fmicb.2022.754225. eCollection 2022. Front Microbiol. 2022. PMID: 35300485 Free PMC article.
-
The inositol 1,4,5-trisphosphate receptor is required to signal autophagic cell death.Mol Biol Cell. 2008 Feb;19(2):691-700. doi: 10.1091/mbc.e07-08-0823. Epub 2007 Dec 12. Mol Biol Cell. 2008. PMID: 18077554 Free PMC article.
-
Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7500-E7509. doi: 10.1073/pnas.1608767113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821730 Free PMC article.
-
Calcium responses to external mechanical stimuli in the multicellular stage of Dictyostelium discoideum.Sci Rep. 2022 Jul 20;12(1):12428. doi: 10.1038/s41598-022-16774-3. Sci Rep. 2022. PMID: 35859163 Free PMC article.
-
The Dictyostelium Model for Mucolipidosis Type IV.Front Cell Dev Biol. 2022 Apr 13;10:741967. doi: 10.3389/fcell.2022.741967. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493081 Free PMC article.
References
-
- Yumura S, Furuya K, Takeuchi I. Intracellular free calcium responses during chemotaxis of Dictyostelium cells. J Cell Sci. 1996;109:2673–2678. - PubMed
-
- Nebl T, Fisher PR. Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J Cell Sci. 1997;110:2845–2853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

