Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice
- PMID: 15745963
- PMCID: PMC2791344
- DOI: 10.1523/JNEUROSCI.5071-04.2005
Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice
Abstract
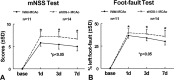
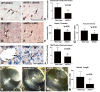
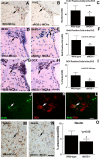
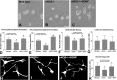
Here, we investigate the effects of endothelial nitric oxide synthase (eNOS) on angiogenesis, neurogenesis, neurotrophic factor expression, and neurological functional outcome after stroke. Wild-type and eNOS knock-out (eNOS-/-) mice were subjected to permanent occlusion of the right middle cerebral artery. eNOS-/- mice exhibited more severe neurological functional deficit after stroke than wild-type mice. Decreased subventricular zone (SVZ) progenitor cell proliferation and migration, measured using bromodeoxyuridine, Ki-67, nestin, and doublecortin immunostaining in the ischemic brain, and decreased angiogenesis, as demonstrated by reduced endothelial cell proliferation, vessel perimeter, and vascular density in the ischemic border, were evident in eNOS-/- mice compared with wild-type mice. eNOS-deficient mice also exhibited a reduced response to vascular endothelial growth factor (VEGF)-induced angiogenesis in a corneal assay. ELISAs showed that eNOS-/- mice have decreased brain-derived neurotrophic factor (BDNF) expression but not VEGF and basic fibroblast growth factor in the ischemic brain compared with wild-type mice. In addition, cultured SVZ neurosphere formation, proliferation, telomerase activity, and neurite outgrowth but not cell viability from eNOS-/- mice were significantly reduced compared with wild-type mice. BDNF treatment of SVZ cells derived from eNOS-/- mice restored the decreased neurosphere formation, proliferation, neurite outgrowth, and telomerase activity in cultured eNOS(-/-) SVZ neurospheres. SVZ explant cell migration also was significantly decreased in eNOS-/- mice compared with wild-type mice. These data indicate that eNOS is not only a downstream mediator for VEGF and angiogenesis but also regulates BDNF expression in the ischemic brain and influences progenitor cell proliferation, neuronal migration, and neurite outgrowth and affects functional recovery after stroke.
Figures


Similar articles
-
Role of endothelial nitric oxide synthetase in arteriogenesis after stroke in mice.Neuroscience. 2009 Mar 17;159(2):744-50. doi: 10.1016/j.neuroscience.2008.12.055. Epub 2009 Jan 6. Neuroscience. 2009. PMID: 19154781 Free PMC article.
-
Endothelial nitric oxide synthase regulates white matter changes via the BDNF/TrkB pathway after stroke in mice.PLoS One. 2013 Nov 13;8(11):e80358. doi: 10.1371/journal.pone.0080358. eCollection 2013. PLoS One. 2013. PMID: 24236179 Free PMC article.
-
Bone Marrow Endothelial Progenitor Cell Transplantation After Ischemic Stroke: An Investigation Into Its Possible Mechanism.CNS Neurosci Ther. 2015 Nov;21(11):877-86. doi: 10.1111/cns.12447. Epub 2015 Sep 19. CNS Neurosci Ther. 2015. PMID: 26384586 Free PMC article.
-
Endothelial Nitric Oxide Synthase-Deficient Mice: A Model of Spontaneous Cerebral Small-Vessel Disease.Am J Pathol. 2021 Nov;191(11):1932-1945. doi: 10.1016/j.ajpath.2021.02.022. Epub 2021 Mar 10. Am J Pathol. 2021. PMID: 33711310 Free PMC article. Review.
-
Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury.Neural Regen Res. 2016 Jan;11(1):174-9. doi: 10.4103/1673-5374.175067. Neural Regen Res. 2016. PMID: 26981109 Free PMC article. Review.
Cited by
-
Targeting eNOS and beyond: emerging heterogeneity of the role of endothelial Rho proteins in stroke protection.Expert Rev Neurother. 2009 Aug;9(8):1171-86. doi: 10.1586/ern.09.70. Expert Rev Neurother. 2009. PMID: 19673606 Free PMC article. Review.
-
Pepsin pretreatment allows collagen IV immunostaining of blood vessels in adult mouse brain.J Neurosci Methods. 2007 Jun 15;163(1):76-82. doi: 10.1016/j.jneumeth.2007.02.020. Epub 2007 Feb 25. J Neurosci Methods. 2007. PMID: 17403541 Free PMC article.
-
Intraarterial transplantation of human umbilical cord blood mononuclear cells is more efficacious and safer compared with umbilical cord mesenchymal stromal cells in a rodent stroke model.Stem Cell Res Ther. 2014 Apr 1;5(2):45. doi: 10.1186/scrt434. Stem Cell Res Ther. 2014. PMID: 24690461 Free PMC article.
-
Emerging treatments for traumatic brain injury.Expert Opin Emerg Drugs. 2009 Mar;14(1):67-84. doi: 10.1517/14728210902769601. Expert Opin Emerg Drugs. 2009. PMID: 19249984 Free PMC article. Review.
-
Ischemia-induced Neuronal Cell Death Is Mediated by Chemokine Receptor CX3CR1.Sci Rep. 2018 Jan 11;8(1):556. doi: 10.1038/s41598-017-18774-0. Sci Rep. 2018. PMID: 29323156 Free PMC article.
References
-
- Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H (2003) Protective role of endothelial nitric oxide synthase. J Pathol 199: 8-17. - PubMed
-
- Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ (1998) Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res 82: 1007-1015. - PubMed
-
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA (2002) BDNF stimulates migration of cerebellar granule cells. Development 129: 1435-1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
