Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli
- PMID: 15743938
- PMCID: PMC1064028
- DOI: 10.1128/JB.187.6.1923-1929.2005
Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli
Abstract
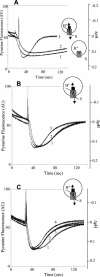
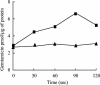
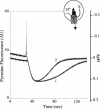
To understand better the mechanisms of resistance-nodulation-division (RND)-type multidrug efflux pumps, we examined the Escherichia coli AcrD pump, whose typical substrates, aminoglycosides, are not expected to diffuse spontaneously across the lipid bilayer. The hexahistidine-tagged AcrD protein was purified and reconstituted into unilamellar proteoliposomes. Its activity was measured by the proton flux accompanying substrate transport. When the interior of the proteoliposomes was acidified, the addition of aminoglycosides to the external medium stimulated proton efflux and the intravesicular accumulation of radiolabeled gentamicin, suggesting that aminoglycosides can be captured and transported from the external medium in this system (corresponding to cytosol). This activity required the presence of AcrA within the proteoliposomes. Interestingly, the increase in proton efflux also occurred when aminoglycosides were present only in the intravesicular space. This result suggested that AcrD can also capture aminoglycosides from the periplasm to extrude them into the medium in intact cells, acting as a "periplasmic vacuum cleaner."
Figures
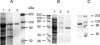
Comment in
-
Vacuuming the periplasm.J Bacteriol. 2005 Mar;187(6):1879-83. doi: 10.1128/JB.187.6.1879-1883.2005. J Bacteriol. 2005. PMID: 15743933 Free PMC article. Review. No abstract available.
Similar articles
-
Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops.J Bacteriol. 2002 Dec;184(23):6490-8. doi: 10.1128/JB.184.23.6490-6499.2002. J Bacteriol. 2002. PMID: 12426336 Free PMC article.
-
Cryo-EM Structures of AcrD Illuminate a Mechanism for Capturing Aminoglycosides from Its Central Cavity.mBio. 2023 Feb 28;14(1):e0338322. doi: 10.1128/mbio.03383-22. Epub 2023 Jan 10. mBio. 2023. PMID: 36625574 Free PMC article.
-
AcrA dependency of the AcrD efflux pump in Salmonella enterica serovar Typhimurium.J Antibiot (Tokyo). 2011 Jun;64(6):433-7. doi: 10.1038/ja.2011.28. Epub 2011 Apr 20. J Antibiot (Tokyo). 2011. PMID: 21505470
-
[The role of cell wall organization and active efflux pump systems in multidrug resistance of bacteria].Mikrobiyol Bul. 2007 Apr;41(2):309-27. Mikrobiyol Bul. 2007. PMID: 17682720 Review. Turkish.
-
AcrAB and related multidrug efflux pumps of Escherichia coli.J Mol Microbiol Biotechnol. 2001 Apr;3(2):215-8. J Mol Microbiol Biotechnol. 2001. PMID: 11321576 Review.
Cited by
-
Novel RpoS-Dependent Mechanisms Strengthen the Envelope Permeability Barrier during Stationary Phase.J Bacteriol. 2016 Dec 28;199(2):e00708-16. doi: 10.1128/JB.00708-16. Print 2017 Jan 15. J Bacteriol. 2016. PMID: 27821607 Free PMC article.
-
Efflux-mediated drug resistance in bacteria: an update.Drugs. 2009 Aug 20;69(12):1555-623. doi: 10.2165/11317030-000000000-00000. Drugs. 2009. PMID: 19678712 Free PMC article. Review.
-
The 3D architecture of a bacterial swarm has implications for antibiotic tolerance.Sci Rep. 2018 Oct 25;8(1):15823. doi: 10.1038/s41598-018-34192-2. Sci Rep. 2018. PMID: 30361680 Free PMC article.
-
Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective.Front Microbiol. 2018 May 28;9:950. doi: 10.3389/fmicb.2018.00950. eCollection 2018. Front Microbiol. 2018. PMID: 29892271 Free PMC article. Review.
-
Vestibules are part of the substrate path in the multidrug efflux transporter AcrB of Escherichia coli.J Bacteriol. 2011 Oct;193(20):5847-9. doi: 10.1128/JB.05759-11. Epub 2011 Aug 19. J Bacteriol. 2011. PMID: 21856849 Free PMC article.
References
-
- Akama, H., T. Matsuura, S. Kashiwagi, H. Yoneyama, T. Tsukihara, A. Nakagawa, and T. Nakae. 2004. Crystal structure of the membrane fusion protein, MexA of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279:25939-25942. - PubMed
-
- Avila-Sakar, A. J., S. Misaghi, E. M. Wilson-Kubalek, K. H. Downing, H. Zgurskaya, H. Nikaido, and E. Nogales. 2001. Lipid-layer crystallization and preliminary three-dimensional structural analysis of AcrA, the periplasmic component of a bacterial multidrug efflux pump. J. Struct. Biol. 136:81-88. - PubMed
-
- Clement, N. R., and J. M. Gould. 1981. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry 20:1534-1544. - PubMed
-
- Davidson, A. L., and H. Nikaido. 1991. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J. Biol. Chem. 266:8946-8951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

